Visionix optovue ifusion80 User manual

User Manual
P/N 580-48483-004 Rev B
optovue ifusion80
iFusion User’s Manual 2 P/N 580-48483-004 Rev B
Publishing Details
iFusion
Please refer to the iVue 100 User’s Manual and the iCam 100 User’s
Manual for full details before attempting to image patients with this
device.
Optovue Inc.
2800 Bayview Drive
Fremont, CA, USA, 94538
Phone: 510-623-8868
Fax: 510-623-8668
www.optovue.com
E-mail: [email protected]m
Item Part Number
iFusion manual 580-48483-004 ECR#02979
iVue 100
iCam 100
Cautions
Federal (U.S.A.) law restricts this device to sale, distribution and use by or
on the order of a physician. Proper procedures and techniques are the
responsibility of the medical professional. It is the operator’s responsibility to
use, check, and maintains this device according to the labels of the product,
accompanying instruction manuals, and any revisions of the labeling or
instructions that may be subsequently issued.
No User serviceable parts, please contact your Optovue, Inc. service
representative. Aucune pièce réparable par l'utilisateur, retourner
l'instrument au fabricant pour réparation.Installation of this equipment is
intended to be performed by Optovue trained service personnel only.
License and use of the iFusion system is intended only
for trained medical personnel in accordance with the
license agreement – all other usage is prohibited –
warranty restrictions and possible claim limitations
apply. All warnings and restrictions that apply to iVue
100 and iCam 100 apply to iFusion combination. Please
read both iCam 100 and iVue 100 manuals prior to using
this configuration.
For Customer Service or Technical Support:
866-941-9240 U.S.
510-743-0985 (U.S. & International)

iFusion User’s Manual 3 P/N 580-48483-004 Rev B
TABLE OF CONTENTS
Table Of Contents ....................................................................................................................3
1. Introduction.....................................................................................................................5
2. Safety Notes.....................................................................................................................7
2.2 Alerts for Danger, Warning, Caution, Important, and Note 10
2.3. Protective Packing Symbols 12
2.4. System Warnings Avertissements du système 13
2.5. Table Handling Instructions 18
2.6. iFusion System Label 27
3 Instrument Description.......................................................................................................29
3.1 iFusion System Configuration 29
3.1.1 iCam 100 Camera Head..............................................................................................29
3.1.2 iVue 100 Scanner Head...............................................................................................29
3.1.3 Computer…… ..............................................................................................................29
3.1.3.1 Data Backup ............................................................................................................29
3.1.4 Joystick/Chin Rest Assembly.......................................................................................29
3.1.5 iShuttle………. ..............................................................................................................30
4 Getting Started ..............................................................................................................32
4.1 Chin Rest…32
4.2 Connecting iCam 100 And iVue 100 To Computer 32
4.3 Starting the System 36
5.0 Patient Imaging............................................................................................................37
5.1 Patient And System Position 37
5.2 Suggested Capture Sequence 38
5.3 Beginning Image Capture 39
5.4 Image Transfer From iCam 40
6. iFusion Overlay...................................................................................................................43
6.1. Overlay Alignment Process 43
6.2 Retina 3-D Scan with Overlay 46

iFusion User’s Manual 4 P/N 580-48483-004 Rev B
6.3 iWellness With iCam Image 47
6.4 3-D Optic Nerve Scan with Overlay 48
6.5 Retina Map with Overlay 49
6.6 Troubleshooting 50
7. Product Specifications ...................................................................................................51
7.1 iVue 100 Scanner & iCam 100 51
Please see individual manuals for details. 51
8. Maintenance..................................................................................................................53
8.1 Routine Care 53
8.1.1 Ocular (Front Objective) Lens and Cornea Lens Cleaning ..........................................53
Material Required:.................................................................................................................53
Method:…...............................................................................................................................53
8.2 Forehead and Chin Rest..............................................................................................53
Material Required:.................................................................................................................53
8.3 Instrument Body Cleaning 54
Index ......................................................................................................................................55

iFusion User’s Manual 5 P/N 580-48483-004 Rev B
1. Introduction
iFusion from Optovue is the combination of the non-mydriatic
fundus camera, iCam 100, and the Spectral Domain Optical
Coherence Tomography (SD-OCT) iVue 100 on a shuttle
platform. The combination is a single retinal imaging system.
iFusion is designed to provide fundus images as well as SD-
OCT images of the patients. The utilization of the iShuttle
combines the heads of both systems on one joystick to
conserve space and improve efficiency.
Please read both the iCam 100 and iVue 100 manual for
safety, warnings and instructions for use before attempting
to use this equipment.
Note: The iFusion Manual is not a modification of the
iCam 100 or iVue 100 manuals. It is a separate manual
which includes additions for iFusion. For specific
details related to iCam 100 or iVue 100 refer to the
User or install manuals.

iFusion User’s Manual 6 P/N 580-48483-004 Rev B
This page intentionally left blank.

iFusion User’s Manual 7 P/N 580-48483-004 Rev B
2. Safety Notes
This instrument has been developed and tested in
accordance with Optovue safety standards as well as
national and international regulatory guidelines to ensure a
high degree of instrument safety. Please observe all safety
notes and information in this manual and on the device
labels. This product contains no material which has a
chemical hazard concern.
Conditions for Proper Instrument Use
1. Always enter patient information first.
2. Prepare patient contact surfaces (forehead and chin
rest according to the requirements of Section 8.0,
Maintenance).
3. Instantly turn off the power switch of the instrument
and disconnect the power cable if uncertain problems
arise.
4. Clean ocular lens frequently to ensure good image
quality (refer to Section 8.0, Maintenance).
5. Adjust power table height properly to ensure patient
comfort during the examination.
6. Align the patient’s head and eye position to the
canthus indicator mark on the chin and forehead rest
assembly.
7. Dim the room lights to allow natural dilation of the
patient’s pupil and to provide a comfortable
visualization of the fixation target without glare.
Note: Chemically induced pupil dilation is not
normally needed.
8. Lock all wheels when not moving table
Unlocked Déverrouillé
Locked. Verrouillé
9. Ensure that when lowering the table the areas
indicated by pinch points are clear. Ensure people
and articles are clear, do not store articles in these
areas.
10. To avoid pinching when raising the chin rest, check
patient head position before turning knob to raise it.
11. The operator should warn people not to sit or stand
on any part of the table, including the base and the
top of the table.

iFusion User’s Manual 8 P/N 580-48483-004 Rev B
Indications for Use
The iFusion connects the iCam (K122572) and iVue
(K121739) devices via a sliding bracket mechanism
(iShuttle), to facilitate switching between the two devices.
The iShuttle provides position adjustment ability of the iCam
or iVue device during use. The iFusion interfaces with the
iCam and iVue devices to enable the operation of the iCam
and iVue devices from one computer unit.
Intended Use
iCam Fundus Camera (K122572) – The iCam is a non-
contact, high resolution digital imaging device which is
suitable for photographing, displaying and storing images of
the retina and external areas of the eye to be evaluated
under non-mydriatic conditions. The iCam takes digital
images of the posterior and external structures of the eye
without the use of a mydriatic agent and is intended for use
as an aid to clinicians in the evaluation, diagnosis and
documentation of ocular health. iCam provides images only
and does not provide any diagnostic, pathological analysis or
classification of ocular health or disease.
AND
iVue 100 with Normative Database (K121739) – The iVue is
a non-contact, high resolution optical coherence tomography
system intended for in vivo imaging, axial cross-sectional,
three dimensional imaging and measurement of anterior and
posterior ocular structures, including retina, retinal nerve
fiber layer, ganglion cell complex (GCC), optic disc, cornea,
and anterior chamber of the eye. The iVue with Normative
Database is a quantitative tool for comparison of retina,
retinal nerve fiber layer, ganglion cell complex, and optic disc
measurements to a database of known normal subjects.

iFusion User’s Manual 9 P/N 580-48483-004 Rev B
Equipment Classification
• Type of protection against electric shock: Class 1
• Degree of protection against harmful ingress of water:
IPX0
• Class of operation: Continuous
• Degree of protection against electric shock of applied
part (chin and forehead rests). Type B
Note: The iFusion is not intended to be used as the
sole diagnostic aid in disease identification,
classification or management. The iFusion provides
data to be used in conjunction with other information,
intended to assist an eye care clinician in determining
a diagnosis. A patient diagnosis is the sole domain of
a licensed eye care clinician.
Remarque : L'instrument iFusion n'est pas destiné
à être utilisé comme seul outil de diagnostic pour
l'identification, le classement ou le traitement des
maladies. Les données produites par le iFusion
peuvent être utilisées de pair avec d'autres
données destinées à aider le clinicien des soins
oculaires à établir un diagnostic. Le diagnostic du
patient est le domaine exclusif du clinicien de
soins oculaires qualifié.

iFusion User’s Manual 10 P/N 580-48483-004 Rev B
2.2 Alerts for Danger, Warning, Caution,
Important, and Note
Refer to User’s Manual.
Reportez-vous au livret du mode d'emploi
Presence of electrical shock hazard.
Voltage inside the instrument. Do not remove the instrument
cover or parts.
General Warning Sign
WARNING indicates a potentially hazardous situation which,
if not avoided, could result in death or serious injury. May be
used to indicate the possibility of erroneous data that could
result in an incorrect diagnosis (does not apply to all
products).
CAUTION indicates a potentially hazardous situation, which,
if not avoided, may result in minor or moderate injury. It may
also be used to alert against unsafe practices. May be used
to indicate the possibility of erroneous data that could result
in an incorrect diagnosis (does not apply to all products).
NOTE is used to call attention to notable information that
should be followed during installation, use, or servicing of
this equipment.
European Conformity Mark for TUV Rheinland
European Notified Body:
TÜV Rheinland LGA Products GmbH
Tillystrasse 290431 Nuremburg Germany
Type B Applied parts.
This instrument complies with the specified requirements to
provide protection against electrical shock, particularly
regarding allowable patient leakage current.

iFusion User’s Manual 11 P/N 580-48483-004 Rev B
Manufacturer
Optovue, Inc.
2800 Bayview Drive,
Fremont, CA., USA, 94538
General mandatory action sign
Authorized European Community Representative
Medical Device Safety Services (MDSS) GMbH
Schiffgraben 41
30175 Hannover, Germany
Serial number
Catalog number / part number
No Sitting. Ne pas s'asseoir.
No Pushing. Ne pas appuyer.
Warning: Crushing of Hands.
Avertissement: Risque d'écrasement des mains.
iFusion User’s Manual 12 P/N 580-48483-004 Rev B
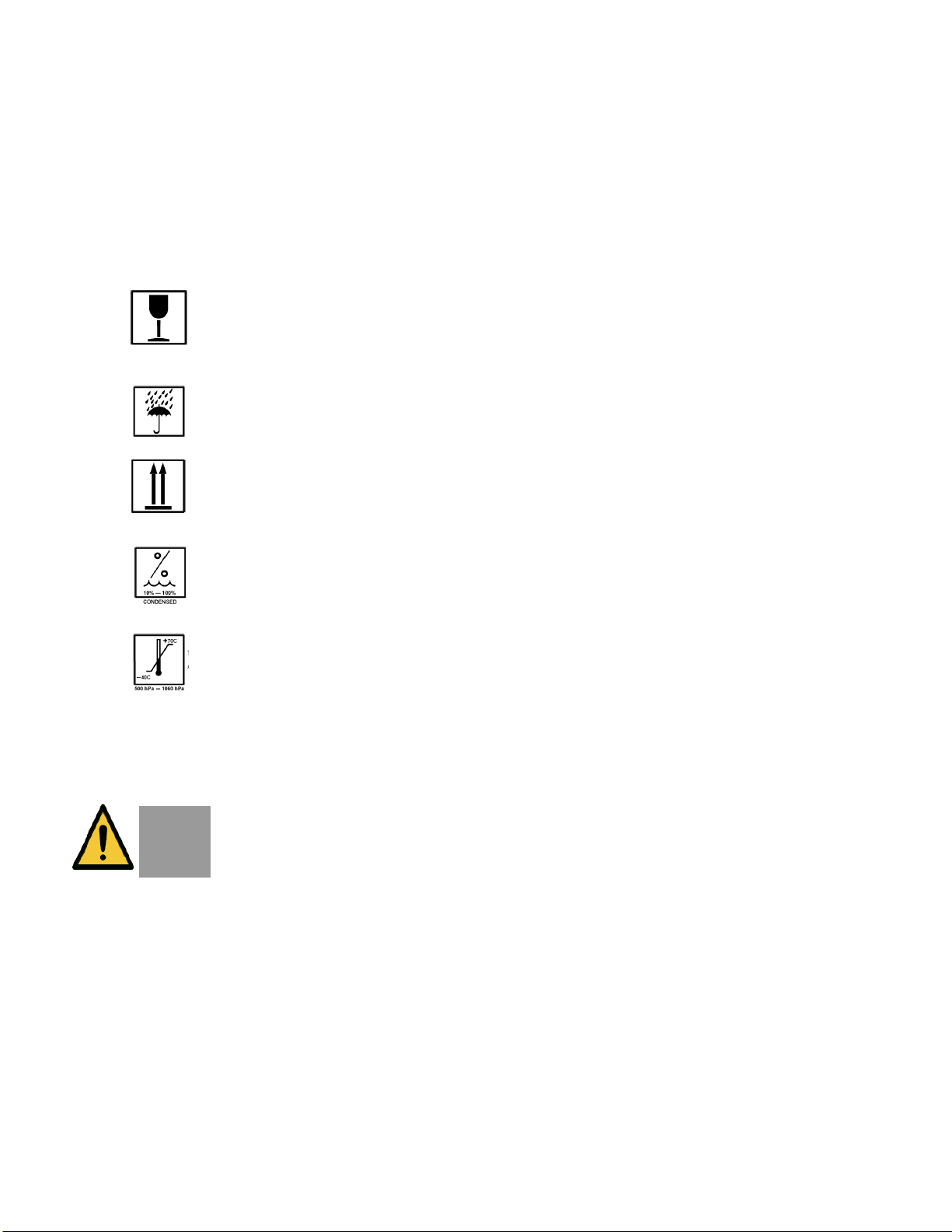
2.3. Protective Packing Symbols
The protective packing symbols specify the handling
requirements and the transport and storage
conditions.
Fragile, Handle with care
Keep Dry
This end up
Relative Humidity (10% to 100%, including
condensation)
Temperature (-40 to 70 deg. C)
Waste Electrical and Electronic Equipment (WEEE)
Recycling Instructions
When determined that the device is ready for disposal, it is
to be recycled following the policies and procedures
reflecting respective country’s requirements. Do not dispose
of device as general waste.
Recycling Label
This symbol is required in accordance with the Waste
Electrical and Electronic Equipment (WEEE) Directive of the
European Union. The presence of this marking on the
product indicates:
1. The device was put on the European market after August
13, 2005
2. The device is not to be disposed of via the municipal
waste collection system of any member state of the

iFusion User’s Manual 13 P/N 580-48483-004 Rev B
European Union. It is very important that customers
understand and follow all laws regarding the proper
decontamination and safe disposal of electrical equipment.
2.4. System Warnings Avertissements du système
WARNING: THE iFusion CANNOT REPLACE CLINICAL
JUDGEMENT AND IS INTENDED TO BE USED ONLY IN
CONJUCTION OTHER CLINICAL TOOLS CONSIDERED
TO BE THE STANDARD OF CARE FOR DIAGNOSIS OF
EYE DISEASE.
AVERTISSEMENT: LE iFusion NE PEUT PAS
REMPLACER LE JUGEMENT CLINIQUE. IL EST
DESTINÉ A ÊTRE UTILISÉ UNIQUEMENT DE PAIR AVEC
LES AUTRES INSTRUMENTS CLINIQUES CONSIDÉRÉS
COMME RESPECTANT LES NORMES EN MATIÈRE DE
POSE DE DIAGNOSTIC POUR LES MALADIES
OCULAIRES.
WARNING: During normal usage of iFusion, software
periodically polls the system status through the USB.
Whenever software detects abnormality in status, it halts
operation and flags error messages to warn users. Upon
seeing the error messages, please exit the application
program, check USB cable connection, and reboot the
system.
The iFusion is not intended to be used as the sole
diagnostic aid in disease identification, classification or
management. The iCam 100 and iVue 100 provide data
to be used in conjunction with other information,
intended to assist an eye care clinician in determining a
diagnosis. A patient diagnosis is the sole domain of a
licensed eye care clinician.
WARNING: No Modification of this equipment is allowed.
WARNING: Do not modify this equipment without
authorization of the manufacturer.
iFusion User’s Manual 14 P/N 580-48483-004 Rev B
WARNING: If this equipment is modified, appropriate
inspection and testing must be conducted to ensure
continued safe use of the equipment.
WARNING: It is recommended that no accessories other
than those specifically called out in this User manual
may be connected to the system. Any customer
accessory equipment connected to the interface ports
must be certified according to the respective IEC
standards (e.g. IEC 60950 for data processing
equipment and IEC 60601-1 for medical equipment).
Furthermore, all configurations shall comply with the
system standard IEC 60601-1:2005. Any person who
connects or installs accessories to the system has the
responsibility to verify the compliance. If in doubt,
consult an Optovue representative.
Avertissement: Il est recommandé de ne pas brancher
sur l'instrument d'autres accessoires que ceux
expressément mentionnés dans ce mode d'emploi. Tout
équipement accessoire client branché aux ports
d'interface doit être certifié selon les normes CEI
respectives (p. ex. la norme CEI 60950 pour le matériel
informatique et la norme CEI 60601-1 pour l'équipement
médical). En outre, toutes les configurations doivent
être conformes à la norme système IEC 60601-1: 2005. Il
incombe à toute personne qui branche ou qui installe
des accessoires à l'appareil de vérifier la conformité de
ces accessoires. En cas de doute, parlez à un
représentant d'Optovue.
WARNING: User Changes to Software or Hardware
The iFusion is a medical device. The software and hardware
has been designed in accordance with U.S., European and
other international medical device design and manufacturing
standards. Unauthorized modification of the iFusion software
or hardware, or any addition or deletion of any application in
any way can jeopardize the safety of operators and patients,
the performance of the instrument, and the integrity of
patient data.
WARNING: Any changes, additions or deletions to
factory installed applications, operating system or
modifications to hardware in any manner VOIDS the
Warranty completely and can cause safety HAZARDS.
Avertissement: Modifications apportées par l'utilisateur
au logiciel ou au matériel informatique.

iFusion User’s Manual 15 P/N 580-48483-004 Rev B
Le iFusion est un instrument médical. Le logiciel et le
matériel informatique ont été conçus conformément aux
normes de conception et de fabrication des appareils
médicaux en vigueur aux É.-U., en Europe et ailleurs. Toute
modification non autorisée du logiciel ou du matériel
informatique du iFusion, ou tout ajout ou suppression d'une
application de quelque manière que ce soit peut présenter
un risque pour la sécurité des opérateurs et des patients, le
fonctionnement de l'instrument et l'intégrité des données des
patients.
Tout changement, ajout ou suppression aux
applications installées en usine et au système
d'exploitation et toute modification au matériel
informatique, de quelque manière que ce soit,
ANNULERONT complètement la garantie et pourraient
présenter un DANGER.
WARNING: Phototoxicity
Avertissement: Phototoxicité
Because prolonged intense light exposure can damage the
retina, the use of the device for ocular examination should
not be unnecessarily prolonged, and the brightness setting
should not exceed what is needed to provide clear
visualization of the target structures.
The retinal exposure dose for a photochemical hazard is a
product of the radiance and the exposure time. If the value of
radiance were reduced in half, twice the time would be
needed to reach the maximum exposure limit.
Du fait que l'exposition prolongée à une lumière intense peut
endommager la rétine, l'utilisation du dispositif pour l'examen
oculaire ne doit pas être inutilement prolongée, et le réglage
de la luminosité ne doit pas dépasser l'intensité nécessaire
pour obtenir une visualisation claire des structures cibles.
La dose d'exposition rétinienne susceptible de présenter un
danger photochimique est le résultat de l'intensité de
radiation et de la durée d'exposition. Lorsque la valeur de
rayonnement est réduite de moitié, le délai nécessaire pour
atteindre la limite d'exposition maximale double.

iFusion User’s Manual 16 P/N 580-48483-004 Rev B
While no acute optical radiation hazards have been identified
for direct or indirect ophthalmoscopes, it is recommended
that the intensity of light directed into the patient’s eye be
limited to the minimum level which is necessary for
diagnosis. Infants, aphakes and persons with diseased eyes
will be at greater risk. The risk may also be increased if the
person being examined has had any exposure to the same
instrument or any other ophthalmic instrument using a visible
light source during the previous 24 hours. This will apply
particularly if the eye has been exposed to retinal
photography.”
Caution: Federal law restricts this device to the sale by or
on the order of a physician or practitioner (CFR 801.109(b)
(1).
« Même si aucune étude ne montre que les rayonnements
optiques des ophtalmoscopes directs ou indirects ont un
effet de toxicité aiguë, il est recommandé de réduire
l'intensité de la lumière dirigée dans l'œil du patient au
niveau strictement nécessaire pour établir le diagnostic. Les
nourrissons, les personnes souffrant d'aphakie (absence de
cornée) et les personnes souffrant d'une maladie oculaire
sont les plus à risque. Le risque peut également augmenter
lorsque la personne examinée a été exposée au même
instrument ou à tout autre instrument ophtalmique utilisant
une source de lumière visible au cours des 24 dernières
heures. Cela est particulièrement vrai lorsque les yeux ont
été exposés à une photographie rétinienne. »
Mise en garde: La loi Fédérale Américaine limite la vente de
cet appareil directement aux médecins ou praticiens ou sur
ordonnance (CFR 801.109 (b) (1)).
No stepping on surface.
Ne pas marcher sur la surface.
Warning: Electrical
Avertissement: Électricité.
ON for part of the Equipment.
Une partie de l'équipement est en marche (« ON »).

iFusion User’s Manual 17 P/N 580-48483-004 Rev B
Alternating Current Courant alternatif.
Contraindications
Contre-indications
This device is not designed, sold or intended for use except
as indicated.
Cet appareil n'est pas conçu ni vendu pour être utilisé de
toute autre manière que celle spécifiée.
ESD Warning:
Prior to assembly, install or interconnection of the iFusion, it
is recommended that any staff (i.e., biomedical engineers
and health care staff) that could touch connectors identified
with the ESD warning symbol undergo ESD training. At
minimum, ESD training should include an introduction to the
physics of electrostatic charge, the voltage levels that can
occur in normal practice and the damage that can be done to
electronic components if they are touched by an operator
who is electro statically charged. Further, an explanation
should be given of methods to prevent build-up of
electrostatic charge, and the how and why to discharge
one’s body to earth or to the frame of the equipment or
system, or bond oneself by means of a wrist strap to the
equipment or system or to earth prior to making a
connection. Finally, staff must be made aware that
accessible pins of connectors identified with the ESD
warning symbol should not be touched with the fingers or
with a handheld tool unless proper precautionary procedures
have been followed.
WARNING: During normal usage of the iCam 100, AC
power fluctuations may cause an error message “unable to
snap a frame.” Upon seeing this error message, please exit
the application program and reboot the system.
Warning Light Sources
IR source: The iCam 100 has continuous IR imaging for the
observation of the patient.
External Fixation light: A continuous light for external
fixation.
External LED: external LED light source for external imaging.
Internal LED flash; intermittent LED light source for the
capture of images.

iFusion User’s Manual 18 P/N 580-48483-004 Rev B
93/42/EEC Medical Device Directive CE Mark.
Indicates this equipment contains Type B applied parts
The iFusion is classified as follows:
• Class I Equipment – Protection against electrical
shock.
• Type B – Degree of protection against electric shock
of applied part (chin and forehead rests).
• IPXO - Degree of protection against ingress of liquids
• Continuous - Mode of operation
2.5. Table Handling Instructions
Directives de manipulation de la table
Moving Parts
Wheel Lock Label.
Étiquette de blocage de roue :
Foot Rest Trapping Warning:
Avertissement de risque de coincement dans le
repose-pied:
Table Up/Down Label: DO NOT PUT FOOT UNDER COMPUTER
Étiquette d'abaissement/relèvement de la table : NE PAS METTRE
LE PIED SOUS L’ORDINATEUR
LOCK
VERROUILLÉ
UNLOCK
DÉVERROUILLÉ

iFusion User’s Manual 19 P/N 580-48483-004 Rev B
Étiquette d'abaissement/relèvement de la
table
Table Up/Down Label
Pinch Warning
LocationsEmplacements pour les
avertissements de risque de
pincement
Prior to moving the iFusion
system, lock iFusion base,
ensure the monitor is
folded down and the table
is lowered to the bottom
position. Unlock the
wheels and follow the table
handling instructions.
Movement of the iFusion
system is accomplished
more easily with two
people, one at each end of
the table
Lock Shuttle &
iBase Joystick
Tighten
monitor
holder
Table at
Lowest
Position
Wheels
Unlocked

iFusion User’s Manual 20 P/N 580-48483-004 Rev B
iFusion System Locked Moving Parts
Lock all wheels when not moving table.
Locked
Disposal:
Dispose of the equipment per local regulations.
Waste Electrical and Electronic Equipment (WEEE)
Recycling Instructions
Déchets d'équipements électriques et électroniques
(DEEE) Instructions de recyclage
When determined that the device is ready for disposal, it is
to
be recycled following the policies and procedures reflecting
respective country’s requirements. Do not dispose of device
as general waste.
Lorsque l'instrument est considéré prêt à l'envoi au rebut, il
doit être recyclé conformément aux politiques et procédures
en vigueur dans le pays. L'instrument à éliminer ne doit pas
être traité comme un déchet ordinaire.
Standard Accessories
Description
Part No.
Quantity
iShuttle with 2-pin connector
500-47989-003
1 pc.
User’s Manual
580-48483-004
1 pc.
Installation Manual
810-48474-002
1 pc.
Table of contents
Other Visionix Laboratory Equipment manuals
Popular Laboratory Equipment manuals by other brands
Topcon
Topcon Compu-Vision CV-5000 Repair manual

MVP
MVP Patriot Megaject Innovator II Operation manual

Auxilab
Auxilab Fugelab-GB10 user manual

ThermoFisher Scientific
ThermoFisher Scientific Thermo Scientific IMP 180 operating instructions

Avaya
Avaya Global Single Port Power over Ethernet Injector... manual

Tektronix
Tektronix P7350SMA instruction manual