Vista phasor 408800 User manual

This manual must be read thoroughly and understood prior to using
the Phasor™ Composite Heating System.
USER’S MANUAL
For use by qualied professionals only
408800
408800-I-V-77-ENG (2)
Inter-Med, Inc.
2200 South St.
Racine, WI 53404-2500 - U.S.A.
Tel.: +1-262-636-9755
Fax: +1-262-636-9760
Toll Free: +1-877-418-4782
www.vista-dental.com
Emergo Europe
Prinsessegracht 20
2514 AP The Hague
The Netherlands
(Patent Pending)
Rx ONLY

TABLE OF CONTENTS
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Welcome to Vista Dental’s PHASOR™ . . . . . . . . . . . . . . . . . . . . . . . . . 3
Contents of the PHASOR™ Kit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Intended Use / Indications for Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
PHASOR™ Set-Up and Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Unpacking the Container . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
LED Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Charging the Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
PHASOR™ Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Inserting Composite . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Composite & Setting Compatibility . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Selecting a Setting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Start Heating Cycle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Cleaning & Disinfection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
Infection Control Measures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Routine Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10-11
Battery Removal & Replacement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Troubleshooting Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Technical Data . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Symbol Identication . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Battery Disposal . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14
Safety Notes, Warnings and Precautions . . . . . . . . . . . . . . . . . . . . . . 14-16
Operating Conditions and Safety Consideration . . . . . . . . . . . . . . 17
Heat Generation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Cool-Down . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Adverse Reactions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Contraindications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
Vista Dental Products Terms and Conditions of Warranty . . . . . 18
Return Policy / Return Restocking Policy . . . . . . . . . . . . . . . . . . . . . . 19
Appendix – Electromagnetic Compatibility . . . . . . . . . . . . . . . . . . . 20-22
and Electrical Safety Information
2

Important!
PLEASE NOTE! Prior to installation and start-up of the device, please read
these instructions carefully. As with all technical devices, the proper function
and safe operation of this device depend on the user’s compliance with the
standard safety procedures as well as the specic safety recommendations
presented in these Operating Instructions.
Introduction
Welcome to Vista Dental’s PHASOR™
Congratulations on your decision to incorporate the PHASOR™ heated
composite delivery system into your practice. This patent-pending device
is the rst of its kind, using NIR (near infrared) technology to rapidly warm
highly lled composite compules. With the touch of a button, PHASOR™
is able to heat composite material to 150°F in seconds, and maintain that
temperature throughout the procedure.
Warming composite signicantly lowers the viscosity of the material resulting
in better adaptation, reduced voids and microleakage, and improved depth of
cure. Materials remain highly sculptable, non-sticky, and easily shaped during
manipulation.
The Vista Dental Products website, www.vista-dental.com, also provides
information on new products, accessories, and educational assistance for
you and your professional sta. If you have any questions regarding the use
of the PHASOR™, please call our customer service department Toll Free at
877.418.4782.
Contents of the PHASOR™ Kit
The Phasor™ is composed of the following:
(1) Handpiece
(3) Removable Nosecones
(1) Stand
(1) Power Supply
(3) Tooth Models
(1) Device Maintenance Kit
(1) Warranty Card
NOTE: ALL COMPONENTS ARE NON-STERILE
3

Intended Use / Indications for Use
Heating and dispensing of dental composite materials.
PHASOR™ Set-Up and Use
Unpacking the Container
No special assistance is required to unpack and assemble the PHASOR™.
If you have questions or concerns, please visit www.vista-dental.com or call
Vista Dental Products at 877.418.4782 (Toll Free).
Packaging should be inspected upon arrival for evidence of shipping damage.
Damaged packaging may indicate the presence of an unsafe product and
the product should not be used until carefully inspected. If the package or
product is damaged, please contact Vista Dental Products at 877.418.4782
(Toll Free) as well as the delivery service to le a complaint.
Introduction
Please reference the image below to familiarize yourself with the PHASOR™.
4
Dispensing Trigger
Battery Compartment
Programming /
Power Button
Charging Port
Removable
Nosecone
Snap-Fit Compule
Heating Chamber

5
Programming / Flow Setting
Battery Level
Indication
Device Activity
Indicator
LED Indicators
The composite gun is designed to display the battery state via the battery level
indicator:
The LED light surrounding the ON/OFF button indicates current charge level
(< 20% charged - Red, > 20% charged - Green).
It is advised to charge the battery when the indication lights are Red.
Charging the Battery
The PHASOR™ battery is partially charged when shipped.
To charge the battery, simply connect the micro-USB charger to the USB charging
port on the device handle and plug the power adapter into a standard outlet.
During battery charging, the device activity indicator light will pulse.
Once charging is complete, the device activity indicator will remain blue.
When unplugged from the charger, the device activity indicator turns o and the
device will enter a sleep mode.

Inserting Composite
Snap a composite compule into the end of the
device. Position the compule so that the tip is
directly under the LED lens.
• Short Compules: position forward
• Long Compules: position back
NOTE: The device will not operate in the absence
of a composite compule.
6
COMPOSITE
BRAND
SETTING 1 SETTING 2 SETTING 3 SETTING 4
3M®*
DENTSPLY®*
Ivoclar®*
Kerr®*
VOCO®*
Coltene®*
Kuraray®*
GC®*
Shofu®
Heraeus Kulzer®*
Tokuyama®*
OPTIMAL Setting Compatible Setting DO NOT USE
CAUTION: Parts of the composite compule may become hot.
Use caution and avoid touching the top of a hot
composite compule.
CAUTION: Do not deviate from the recommended settings.
Composite & Setting Compatibility
For a complete list of compatible compules and their ideal setting
visit www.vista-dental.com/phasor
PHASOR™ Set-UpPHASOR™ Set-Up
PHASOR™ Operation

7
Selecting a Setting
Press & hold to enter programming mode.
This is indicated by ashing yellow lights.
Short press to toggle between settings.
Press & hold to exit.
Device will beep 3 times and shut o.
Your setting is now memorized as default.
1.
2.
3.
4.
Reference setting compatibility chart to determine
the appropriate setting per composite brand.
CAUTION: Do not deviate from
the recommended settings.
Start Heating Cycle
Short press to start/stop heating cycle.
The blue lights will ash while heating.
Setting 1-3: 45 seconds
Setting 4: 70 seconds
The blue lights will turn solid when
heating cycle is complete.
The device will automatically shut o after
3 minutes of sustained heating.

8
Cleaning & Disinfection
The device may be wiped down with a standard disinfectant wipe.
Never spray disinfectant directly onto the device.
Black nosecone is removable and autoclavable.
Between uses, check the lens to ensure it is clean.
A dirty lens will decrease performance.
CAUTION: Always use a
protective barrier.
The PHASOR™ is provided non-sterile. There are no special accessories needed
to sterilize the PHASOR™ heated composite delivery system.
Quaternary ammonium compound products are recommended (containing 20%
alcohol or less). Wipe, do not spray, solution onto the unit. Prevent liquids from
entering openings on the composite gun unit.
DO NOT AUTOCLAVE HANDPIECE.
REMOVABLE NOSECONES CAN WITHSTAND AUTOCLAVE - (132°C / 0.22mPA for 3 minutes).

9
CAUTION:
• DO NOT immerse the unit or unit parts in solutions. Use of solutions
other than those recommended may damage plastic parts and will void
product warranty.
• DO NOT use abrasive material such as scouring powder, organic solvents,
or solvent-based cleaning uids. In case of severe contamination, gently
clean the device by using diluted alcohol.
• Store the device in the box if it is not to be used for an extended
period of time.
Infection Control Measures
CAUTION: To prevent cross-contamination, a disposable plastic sleeve must
be used over the PHASOR™ with each use. A low-density polyethylene plastic
disposable barrier covers the nose cone of unit and provides a hermetically
sealed barrier between the handpiece and patients. The disposable barrier
limits patient-to-patient contamination. Discard used barrier sleeves after
each patient.

10
Routine Maintenance
Between uses, check the lens to ensure it is clean.
A dirty lens will decrease performance.
1. Insert the hex key provided into
the trigger bolt.
2. Rotate counterclockwise and
remove the trigger bolt once loose.
3. Slide the trigger and spring assembly
apart from the device.
When reassembling, ensure spring
is inserted into the small opening
in the trigger space.

11
4. Tilt the device forward to allow the
plunger to slide forward and be
removed from the device.
6. Thoroughly clean the plunger
space and LED lens to remove
any composite residue.
NOTE:
• Alcohol may be used
as a cleaning solution.
• DO NOT insert the brush
past the end of the bristles.
5. With the
nosecone
removed, clean
the device with
the provided
brush.

12
Troubleshooting Guide
If the suggested solutions do not rectify the problem, please call
Vista Dental 877.418.4782 (Toll Free).
Problem Possible Solution
Composite gun will not turn on 1. Check the unit’s battery indication light.
If red or no light, charge the unit.
2. Remove the battery and check orientation.
Reinsert into the device in proper orientation.
1. Verify the unit is charged.
2. Verify that an appropriate temperature
is selected.
3. With the handpiece turned o, and
battery removed, inspect the lens for
residual composites.
4. Check expiration date of composite.
1. Inspect the USB charging port of any
foreign objects that could interfere
with a proper connection.
2. Remove the battery and check orientation.
Reinsert into the device in proper orientation.
3. Make sure the battery is properly inserted
in the handpiece.
4. Make sure the charger is plugged in and
and verify the outlet is receiving power.
1. Adjust ow setting to low or universal
ramp setting.
Composite gun is not heating
Battery will not charge
Composite compules are melting
Battery Removal & Replacement
To remove battery compartment door, press the release button and separate
from device by pulling.
Remove the old battery and replace with the new battery, ensuring that
+/- indicators are properly aligned as indicated on the battery door.

Technical Data
13
Charger Input: 100-240 VAC, 50-60 Hz
Nominal Consumption: 6W max
Manufacturer: GlobeTek INC.
Model: GTM46101-1005-USB
Mass: 50g
Classication: Protection class II,
Dimensions without blade or cable (LxWxH):
41mm x 71mm x 31.5mm
Handpiece Battery: 3.6 V nominal, 3000mAh Li-ion,
10.8 Wh
Battery Pack Manufacturer:
LG CHEM LTD.
Battery Pack Model: LG-HG2-18650-INR
Dimensions (LxWxH): 150mm x 120mm x 25mm
Operating Conditions
Operating Time:
Approximately 2 hrs. fully charged.
Approximately 15 heat cycles on high setting.
Time to Charge Empty Battery Pack:
Approximately 3 hrs
Classication: Type BF,
Intermittent Operation: The device has been
designed solely for short-term operation.
Temperature: 10°C - 40°C (59°F - 104°F)
Atmospheric Pressure: 697hPa - 1013hPa
10°C
40°C
Transport and Storage Conditions Temperature: -20°C − 40°C (-4°F − 104° F)
-20°C
40°C
Relative Humidity: 30% - 90% (non condensing)
30%
90% (max)
Atmospheric Pressure: 500hPa - 1400hPa
500 hPa
1400 hPa
Mass: 153g
Technical Information Phasor™ Composite Gun
697 hPa
1013 hPa

14
Symbol Identication
Description for additional symbols.
Battery Disposal
Batteries contain toxic material and should not be disposed of in landlls
or incinerators. Dispose of depleted batteries as directed by your local solid
waste handling regulations. To dispose of the battery in North America, we
recommend www.call2recycle.com to locate a recycling facility near you.
Safety Notes, Warnings and Precautions
Read all instructions before operating this unit. The PHASOR™ heated
composite delivery system emits high intensity light waves and must only be
used as indicated in this manual.
Serial Number
Manufacturer
Manufacturing Date
Class II Medical
Electrical Equipment
Type BF Patient
Applied Part
Keep Dry
Part Number
Do not reuse
Warning / Caution
Temperature
Limitation
Humidity Limitation
Pressure Limitation
Batch Code / Lot
Number
European
Representative
Do not use if seal
or packaging is
compromised
Autoclavable up to the
temperature specied
Consult instructions
for use
This symbol is a
mandatory marking
for devices entering
the European market
to indicate conformity
with the essential
health and safety
requirements set out
in European Directives
CAUTION: U.S.
federal law restricts
this device to sale by
or on the order of a
dental professional.
This symbol refers to the
special disposal of
electrical and electronic
devices in EU countries.
Please do not discard
this device in household
garbage. Check the
proper means of
disposal in your country
at your community
recycling, waste center
or at your dealer. Take
care to dispose of
properly.

15
WARNING
• The user should test the product before use to ensure proper
functionality. Test each ow setting with the tooth models provided.
• As with any heavily used medical device, the user needs to
ensure a functional backup is readily available.
• DO NOT keep or position near ammable materials, or
materials that could combust.
• DO NOT look directly into the IR light output when the device is on.
• DO NOT insert ngers, instruments, or other objects into the
handpiece when the battery pack is removed.
• DO NOT autoclave the handpiece, battery, charging cord, or stand.
• ONLY heat composite starting at room temperature.
• DO NOT heat composite if it is already at an elevated temperature from
use in the PHASOR™ or other warming devices.
• ONLY USE replacement batteries provided by the manufacturer.
• ONLY DISPENSE composite after warming. Not intended to dispense
composite at room temperature or below.
The PHASOR™ composite gun is a medical device which is subject to IEC
60601-1 (EN 60601-1) and EMC directives IEC 60601-1-2 (EN 60601-1-2)
Edition 4.0, as well as the 93/42/EEC Medical Device Directive. The composite
gun complies with the relevant EU regulations.
The device has been shipped from the manufacturer in a safe and technically
sound condition. In order to maintain this condition and to ensure risk-free
operation, the notes and regulations in these Instructions for Use have to be
observed. To prevent damage to equipment and risk for patients, users and
third parties, the following safety instructions have to be observed.

16
CAUTION
•U.S. Federal law restricts the sale of this device by or on the order of a
healthcare professional. Use of the device is restricted to qualied
and trained personnel only in accordance with the operation
instructions. The manufacturer assumes no liability for any damage
arising from any other or improper use of this device.
• Only use the charger which is provided with the device. The use of
any other charger can result in damage to the device.
• Condensation resulting from the device being transferred from a
cold to a warm environment may be a potential risk. Never begin
operating the device until it has reached the ambient temperature.
• Use only components and accessories listed in the instructions
associated with the device. Failure to do so will void the warranty,
may decrease the performance, and may lead to unsafe operation.
• In order to avoid electric shock, do not introduce any objects into the
device or remove the device enclosure.
• Should you have any reason to suspect the safety of the device to be
compromised, the device must be taken out of operation and labeled
accordingly to prevent third parties from inadvertently using a
possibly defective device. Safety may be compromised, e.g., if the
device malfunctions or is noticeably damaged.
• Keep solvents, ammable liquids, and sources of intense heat away
from the device as they may damage the plastic housing of the
device, the seals, or the operating buttons.

17
Operating Conditions and Safety Consideration
Heat Generation
The PHASOR™ heated composite delivery system has been designed not to
overheat to the point of discomfort or injury during standard operating durations.
However, care should be taken to allow the product to completely cool between
uses (approximately two-three minutes), to ensure overheating does not occur.
Cool-Down
PHASOR™ features a built in fan which will turn on automatically during operation.
The fan will remain on after operation until the device reaches a lower temperature.
Adverse Reactions
There are no known adverse reactions.
Contraindications
PHASOR™ should not be used for any application outside of composite resotrations.
• DO NOT heat composite if it is already at an elevated temperature from
use in the PHASOR™ or other warming devices.
• ONLY DISPENSE composite after warming. Not intended to dispense
composite at room temperature or below.

18
Vista Dental Products Terms and Conditions of Warranty
The Phasor™ handpiece is warranted to be free from defects under normal
usage conditions for one (1) year of its date of delivery; the batteries for
one (1) year. There is no warranty, expressed or implied, of merchantability
or tness. The manufacturer’s sole obligation under this warranty is to opt
to either repair or replace the defective part(s) or product. If service must
be performed to correct a defect, then the manufacturer will provide the
service at its factory according to the mutual agreement made in advance.
The manufacturer and its distributors will not accept the return of the
product unless the return is authorized and shipped in accordance with the
manufacturer’s instructions. Contact the local representative of the distributor,
or if purchased directly from the manufacturer for shipping instructions, a
return authorization number, and ARS shipping label. There is no warranty,
remedy or condition, expressed or implied, except as provided herein. The
warranty and remedies contained herein are made by the manufacturer to the
rst buyer for dental use and are in lieu of all other agreements (expressed or
implied), liabilities or remedies for breach of warranty. Vista Dental Products
shall not be liable for consequential or incidental damages. No person or
distributor is authorized to modify the terms of this warranty.
This warranty is void if any defect is caused by conditions beyond the
manufacturer’s control, including acts of God, damage resulting from
mishandling, neglect, misuse, improper maintenance, accident or alteration/
repair by anyone other than the manufacturer. The buyer assumes all liability
for any damage caused by improper use of the product. The manufacturer
assumes no liability for the user’s failure to follow the instructions contained
in this manual.

19
Return Policy
Vista Dental Products will accept for return previously purchased merchandise
which is suitable for resale or was shipped in error by Vista Dental Products.
Merchandise suitable for resale requires current labeling and unopened
non-soiled packaging.
All returns must have prior approval and must be shipped“prepaid” along with
a return authorization form and a copy of the original invoice. Any products
returned that are discontinued, dated, damaged, or opened could be denied
credit or assessed a higher return fee.
Equipment cannot be returned without written authorization from Vista Dental
Products. Merchandise returned for credit must be received by Vista Dental
Products within 30 days of the original invoice date.
Return Restocking Policy
Any equipment returned within 30 days from the date of the original
shipment from Vista Dental Products may not be assessed a restocking fee
as long as the merchandise has current labeling and unopened non-soiled
packaging. Unopened equipment returned within 31-60 days from the date
of the original shipment from Vista Dental Products requires a restocking fee
of 25% of the purchase price, including shipping and handling charges. Any
equipment returned after 60 days from date of the original shipment from
Vista Dental Products will not be restockable for credit.
• Special orders are not suitable for resale and therefore not
returnable for credit.
• Claims for lost or damaged shipments should be led immediately
with the carrier.
• Claims for overage, shortage, and/or internal damage must be
made to Vista Dental Products within 10 days of receipt of goods.
30 days
21-59 days
60+ days
15%
25%
Not Returnable

20
Appendix – Electromagnetic Compatibility and Electrical
Safety Information
The PHASOR™ heated composite delivery system is tested according to IEC 60601-1-2,
Edition 4.0.
Medical electrical devices are subject to particular preventive action according to
EMC rules and must be installed and operated according to the EMC guidelines in the
accompanying documents.
Guidance and Manufacturer‘s Declaration – Electromagnetic Emission
The following tables are guidelines according to the 4th edition of the medical standard
IEC 60601-1-2.
PHASOR™ heated composite delivery system is intended for use in the electromagnetic
environment specied below. The customer or the user of the PHASOR™ heated
composite delivery system should assure that it is used in such an environment.
Guidance and Manufacturer‘s Declaration – Electromagnetic Immunity
The PHASOR™ heated composite delivery system is intended for use in the
electromagnetic environment specied below. The customer or the user of the
PHASOR™ heated composite delivery system should assure that it is used in such an
environment.
PHASOR™
PHASOR™
PHASOR™
PHASOR™
PHASOR™
PHASOR™
PHASOR™
PHASOR™
Table of contents
Other Vista Dental Equipment manuals
Popular Dental Equipment manuals by other brands
Durr
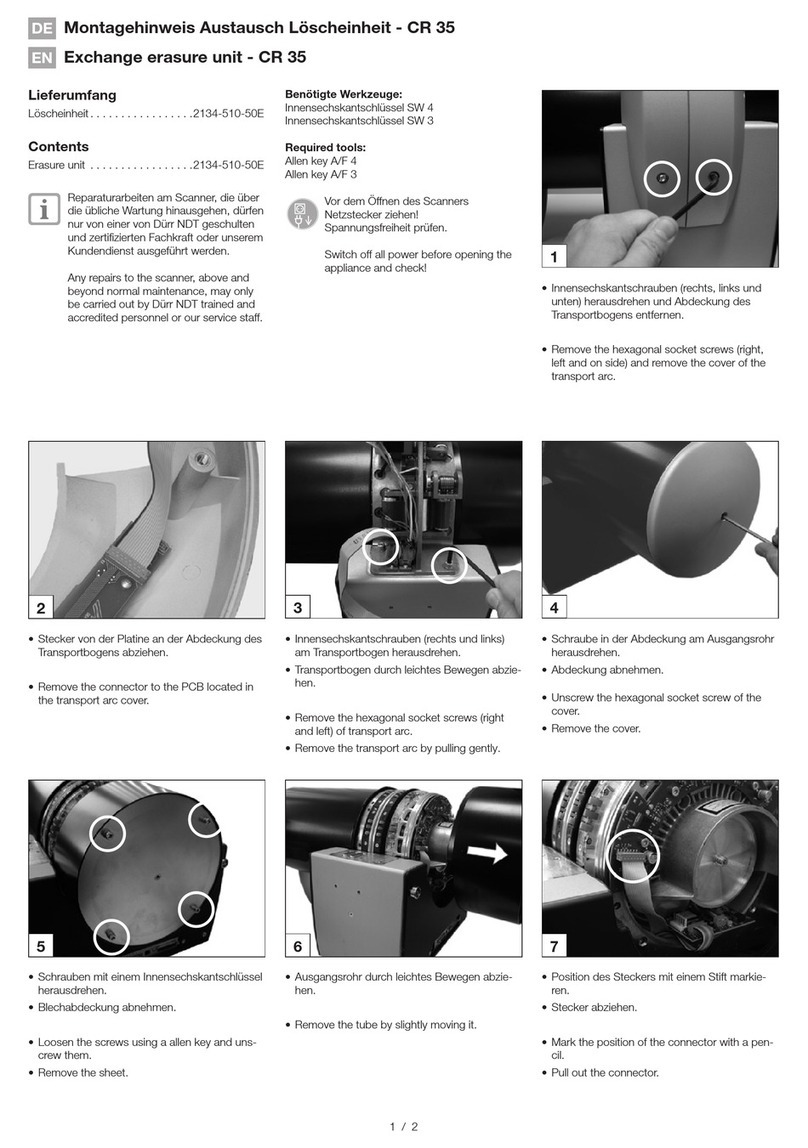
Durr 2134-510-50E quick start guide

NSK
NSK TiMax nano15LS Operation manual

Dentsply Maillefer
Dentsply Maillefer X-SMART manual

BG Light
BG Light BLUEDENT SMART cordless Operating instructions manual

CMS Dental
CMS Dental FlashMax P3 Module Replacement Instructions

HAGER WERKEN
HAGER WERKEN PRAXIPOL PS II Instructions for use