CAUTION CAUTION
* Warning: This device is not AP/APG equipment. Do not use the device where flammable
anesthetic are present, or in environments mixture wi h air of with oxygen or nitrous oxide.
* The device contains sensitive electronic components.To avoid measurement errors, avoid taking
blood pressure measurements near a strong electromagne ic field radiated interference signal
or electrical fast transient/burst signal.
* Wireless communication equipment, such as wireless home network devices, mobile phones,
cordless telephones and their base stations, walkie-talkies may cause interference that may
affect he accuracy of measurements. A minimum distance of 1 foot (30 cm) should be kept from
such devices during a measurement.
* You can use this device to take your own measurement, no third-party operator is required.
* Please use the device under the environment which is provided in the user manual. O herwise,
the performance and lifetime of the device will be impacted and reduced.
* The device may require up to 30 minutes to warm up / cool down from the minimum / maximum
storage temperature before it is ready for use.
* Warning: Excessive cuff tube lengths could cause strangulation if you don't manage them
properly.
* Warning: Do not touch output of the batteries/adapter and the user simultaneously.
* Adapter is specified as a part of ME EQUIPMENT.
* Warning: The power cord is considered the disconnect device for isolating this equipment from
supply mains. Do not position the equipment so that it is difficult to reach or disconnect.
* The blood pressure monitor, its adapter, and the cuff are suitable for use within the patient
environment.
* Warning: Do not use this device if you are allergic to polyester, nylon, or plastic.
* Warning: Only use accessories approved by manufacturer. Using unapproved accessories
might cause damage to the unit and injure users.
* Warning: If you experience discomfort during a measurement, such as pain in the arm or other
complaints, press the Power button immediately to release the air from the cuff.
* No calibration is required within two years of reliable service.
* Do not attempt to repair the unit yourself if it malfunctions. Only have repairs carried out by
authorized service centers.
* At he request of authorized service personnel, circuit diagrams, component part lists,
descriptions, and calibration procedures will be made available by the manufacturer or
distributor.
* It is recommended that he performance should be checked after repair, maintenance, and
every two years of use, by retesting the requirements in limits of the error of the cuff pressure
indication and air leakage (testing at least at 50 mmHg and 200 mmHg).
* Warning: Do not use the device while under maintenance, or being serviced.
* Store your device, cuff and adapter in a clean and dry place, protect it against extreme moisture,
heat, lint, dust and direct sunlight. Never place any heavy objects on it.
* Make sure the rubber tube of the cuff is not squeezed, stretched, or kinked during storage.
* Warning: Keep the device, cuff, and batteries away from children as they may pose a risk of
choking or strangulation if used improperly.
* Clean both device and cuff with a soft, dry cloth. If necessary use a dampened cloth and natural
detergent. Do not use alcohol, benzene, or other harsh chemicals.
* Do not wash the cuff in a washing machine or dishwasher!
* The service life of the cuff may vary by the frequency of washing, skin condition, and storage
state. The typical service life is 10000 times.
* Dispose of accessories, detachable parts, and the device according to the local guidelines.
* This device is intended for indoor, home use.
* This device is not intended for public use.
* This device is portable, but it is not intended for use during patient transport.
* This device is not suitable for continuous monitoring during medical emergencies or operations.
* This device is intended for no-invasive measuring and monitoring of arterial blood pressure. It is
not intended for use on extremities other than the arm, or for any purpose other than obtaining a
blood pressure measurement.
* This device is for adults. Do not use this device on neonates or infants. Do not use it on children
unless otherwise instructed by a medical professional.
* Do not use on the women in pregnant, including pre-eclamptic, patients.
* The device is not suitable for use on patients with implanted, electrical devices, such as cardiac
pacemakers, defibrillators.
* The effectiveness of this device has not been established for use:
-on users with common arrhythmias such as atrial or ventricular premature beats or atrial
fibrillation,
-on users with peripheral arterial disease,
-on users undergoing intravascular therapy, or with arteriovenous (AV) shunt.
Consult a medical professional before use.
* Do not use this device for diagnosis or treatment of any health problem or disease. Contact your
physician if you have or suspect any medical problem. Do not change your medications without
the advice of your physician or health care professional.
* If you are taking medica ion, consult your physician to determine the proper time to measure
your blood pressure.
* This device may be used only for the intended use described in this manual, the manufacturer
shall have no liability for any incidental, consequential, or special damages caused by misuse or
abuse.
* Report any unexpected operation or events to the manufacturer.
* Do not apply the cuff on an arm that has an intravenous drip or a blood transfusion attached.
* Warning: Do not kink, fold, stretch, compress, or otherwise deform the tube during measuring, as
the cuff pressure might continuously increase, which could prevent blood flow and result injury.
* Warning: Taking blood pressure measurements too frequently could disrupt blood circulation and
cause injuries.
*Warning: Do not apply cuff to areas on patient where skin is delicate or damaged. Check cuff site
frequently for irritation.
* Warning: Do not place the cuff on the arm of a person whose arteries or veins are undergoing
medical treatment, i.e. intra-vascular access or intra-vascular therapy or an arteriovenous (A-V)
shunt, which could disrupt blood circulation and cause injuries.
* Do not place the cuff on the arm on the same side of a mastectomy (especially when lymph
nodes have been removed). it is recommended to take measurements on the unaffected side.
* Do not wrap the cuff on the same arm to which another monitoring device is applied. One or both
devices could temporarily stop functioning if you try to use them at the same time.
* Please check that the operation of the device do not result in prolonged impairment of patient
blood circulation.
* Warning: On the rare occasion of a fault causing the cuff to remain fully inflated during
measurement, loosen and remove the cuff immediately. Prolonged high pressure applied to the
arm (cuff pressure >300 mmHg or constant pressure >15 mmHg for more than 3 minutes) might
lead to bruising and discolored skin.
* Warning: Do not use this device with high-frequency (HF) surgical equipment at the same time.
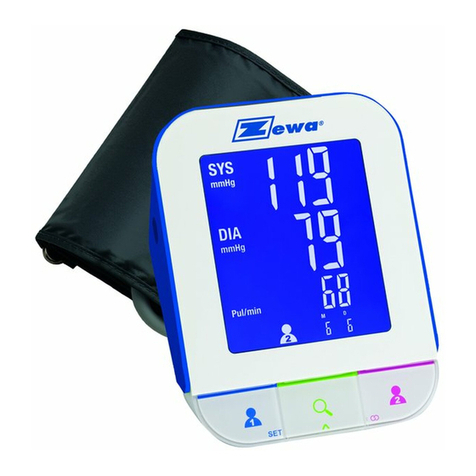
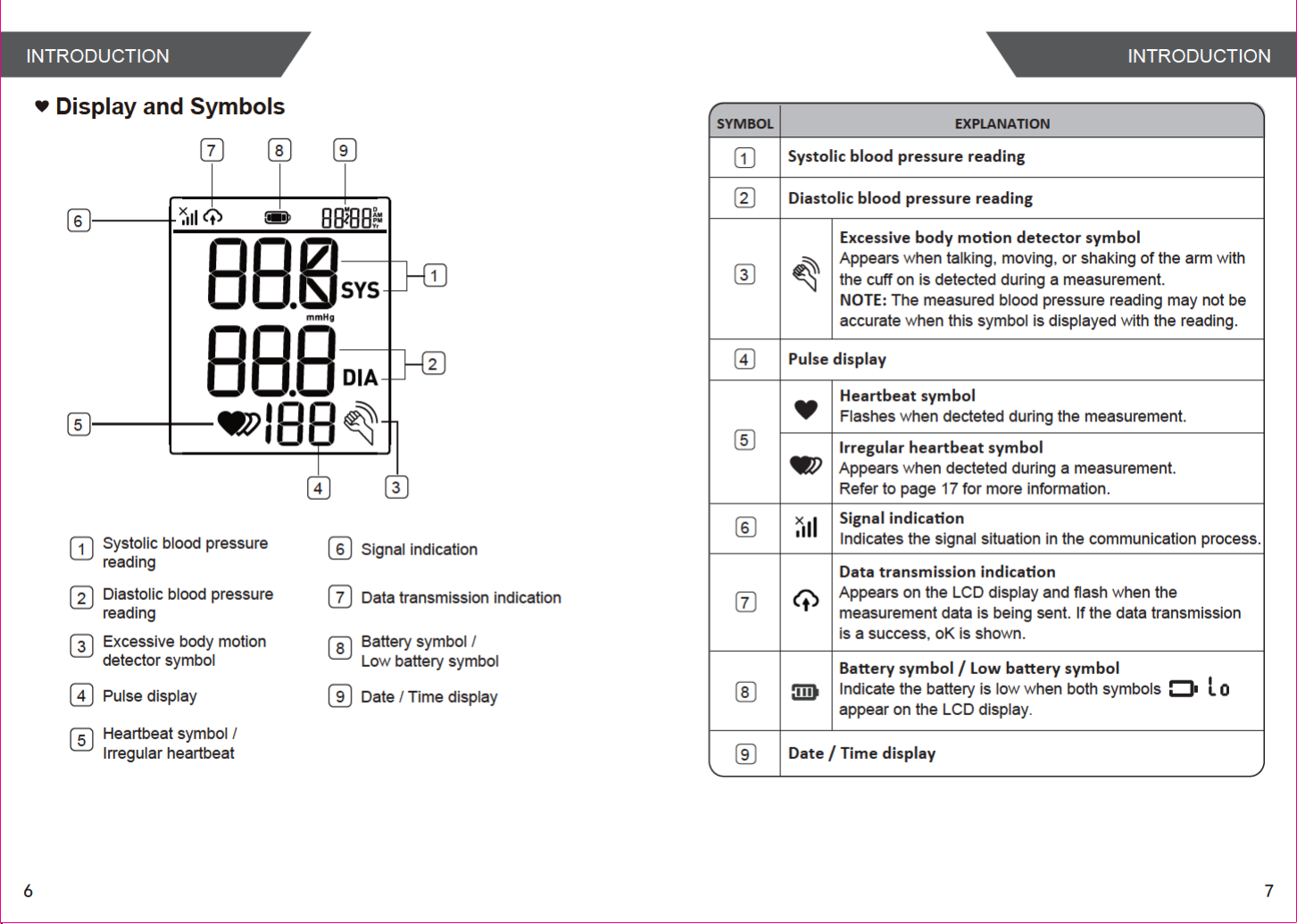
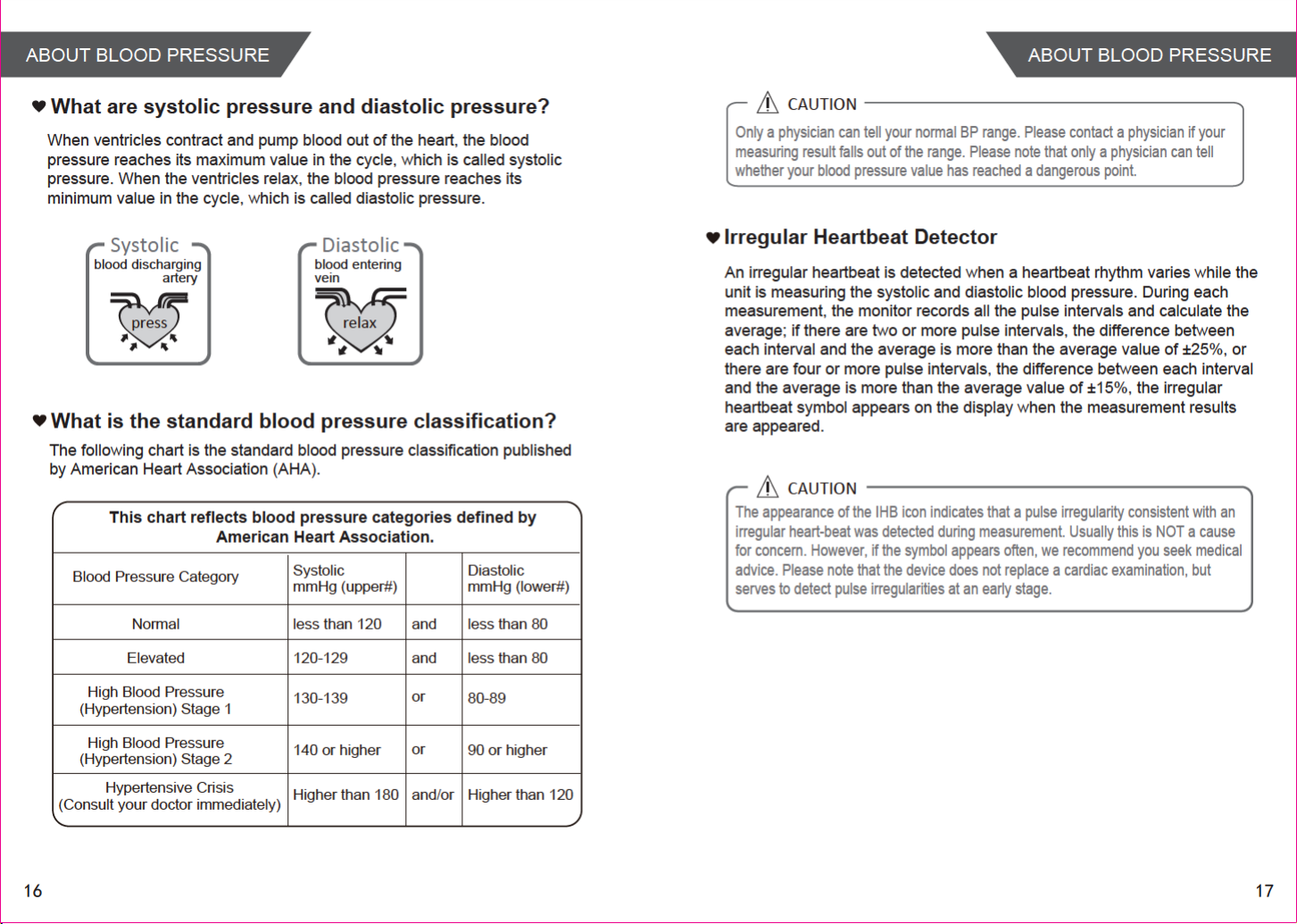
INTRODUCTIONINTRODUCTION
45