9650-0803-01 Rev. B Propaq M Operator’s Guide i
Table of Contents
Chapter 1 General Information
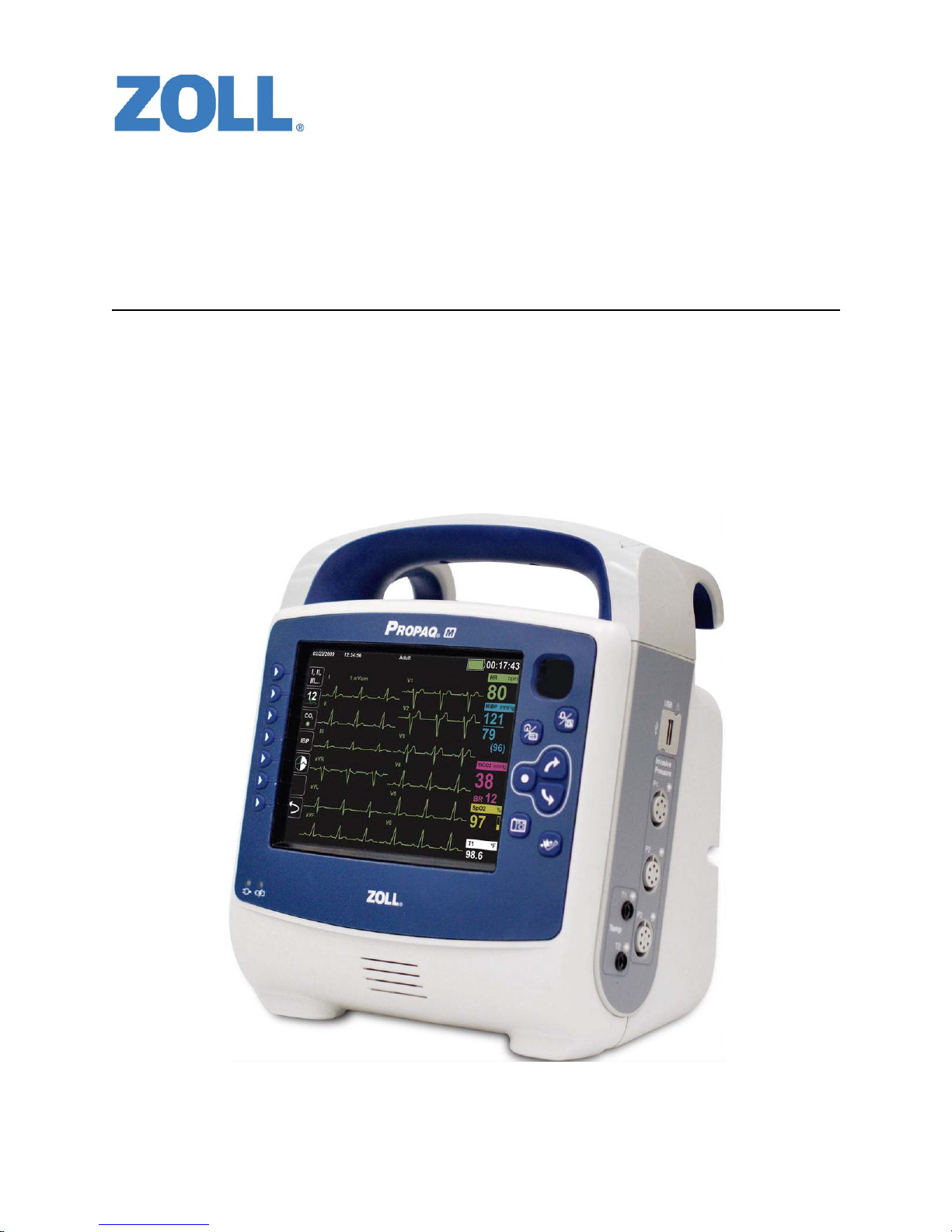
Product Description ............................................................................................................1-1
Propaq M Optional Features .......................................................................................1-2
How to Use This Manual.....................................................................................................1-2
Operator’s Guide Updates..................................................................................................1-2
Unpacking...........................................................................................................................1-2
Symbols Used on the Equipment .......................................................................................1-3
Conventions........................................................................................................................1-5
Propaq M Indications for Use..............................................................................................1-6
ECG Monitoring ..........................................................................................................1-6
Non-Invasive Blood Pressure Monitoring ...................................................................1-6
Temperature Monitoring .............................................................................................1-6
Sp02 Monitoring ..........................................................................................................1-6
Respiration Monitoring ................................................................................................1-7
CO2 Monitoring ...........................................................................................................1-7
Invasive Pressure Monitoring .....................................................................................1-7
Propaq M Product Functions ..............................................................................................1-7
ECG Monitoring ..........................................................................................................1-7
Batteries ......................................................................................................................1-8
Ready For Use (RFU) Indicator ..................................................................................1-9
Warnings...........................................................................................................................1-10
General .....................................................................................................................1-10
ECG Monitoring ........................................................................................................1-11
Pulse Oximeter .........................................................................................................1-11
Noninvasive Blood Pressure .....................................................................................1-12
IBP ............................................................................................................................1-13
CO2 ..........................................................................................................................1-13
Respiration ................................................................................................................1-13
Ferromagnetic Equipment ........................................................................................1-13
Battery ......................................................................................................................1-14
Operator Safety ........................................................................................................1-14
Patient Safety ...........................................................................................................1-14
Cautions............................................................................................................................1-15
Restarting the Monitor.......................................................................................................1-16
Notification of Adverse Events ..................................................................................1-16
Software License ..............................................................................................................1-16
Service..............................................................................................................................1-17
The ZOLL Serial Number..................................................................................................1-18