6
Welcome to the BioCAM X platform and thank you for your purchase. The BioCAM X platform is among
the most advanced systems for managing your experiments on the emerging generation of high-den-
sity CMOS-MEAs. This manual has been written to help you to take advantage of all functionalities
provided by your BioCAM X. Be sure to read this manual thoroughly, and to keep it handy when using
the BioCAM X platform.
Before any use of your BioCAM X platform, please read the “Precautions” on page 15, which contains
relevant information to preserver your BioChips and BioCAM X from possible damages.
MEA technology
Amongthedierentmethodologiesusedforelectrophysiologicalmeasures,metalmicroelectrodesin-
tegrated on-chip can provide multisite measures of extracellular signals with a high signal-to-noise ratio.
In addition, by applying voltage or current stimuli to the same microelectrodes it is possible to depolar-
ize cells or tissues, thus establishing bi-directional interfaces. As established over several decades of
research, both sensing and actuating performances of microelectrodes can be applied to study a wide
range of electrogenic cells and tissues, including neuronal and cardiac preparations.
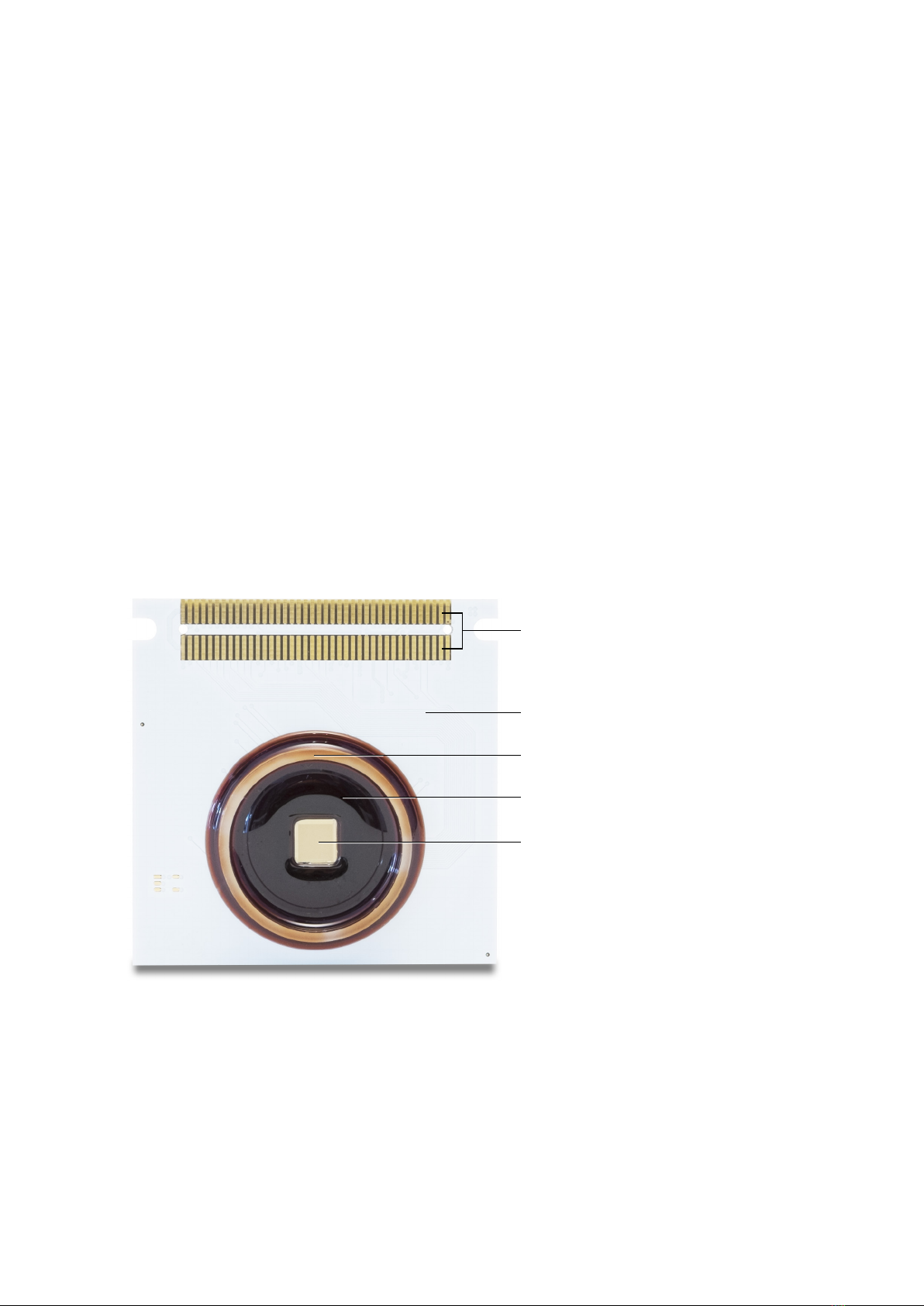
Conventionalmicroelectrodearrays(MEAs)arebio-sensingchipsrealizedbymeansofthin-lmtech-
nologies and do not integrate on-chip any microelectronic circuit. Therefore, conventional MEAs are
passive devices made on silicon, glass or polymeric substrates. Each microelectrode can be made by
dierentmaterials(e.g.Pt,IrOx,TiN)anditisindividuallywiredon-chipandconnectedtoanexternal
amplieranddataacquisition(DAQ)instrument.DuetotheneedofthisMEAtechnologyofindividually
routing on-chip each electrode, space constraints and wiring encumbrance impede the realization of
dense and large electrode arrays. Thus, for conventional MEAs the typical electrode pitch is in the range
of 100 µm and the array includes from 60 up to 256 microelectrodes. In addition, given the distance
betweentheelectrodesandtheo-chipampliersandtheuseofinterconnectingwires,conventional
MEAs are subjected to inductive coupling noise.
CMOS-MEA technology
The electrode density and array sizes can be increased by changing the technology used to realize
microelectrode arrays (MEAs). High-density MEAs are realized with complementary metal–oxide–semi-
conductor technology (CMOS), as it is done for microelectronic devices (e.g., computer microproces-
sors) and light-imaging devices (e.g., camera sensor), and with post-processing methods to optimize the
electrode performances.
Briey,theCMOStechnologyallowstorealizeactiveelectrode-pixelsthatintegrateinsmallareasofa
fewsquaremicrometerselectrodes,ampliersandsignalconditioningcircuitsin-pixel,justunderneath
eachelectrodesite.On-chip,additionalamplicationstages,multiplexingandhigh-speedaddressing
circuits are provided.
In particular, the circuit architecture of 3Brain’s BioChips is based on the Active Pixel Sensor concepts,
as commonly used for light imaging CMOS cameras, and was designed to allow full array recordings at