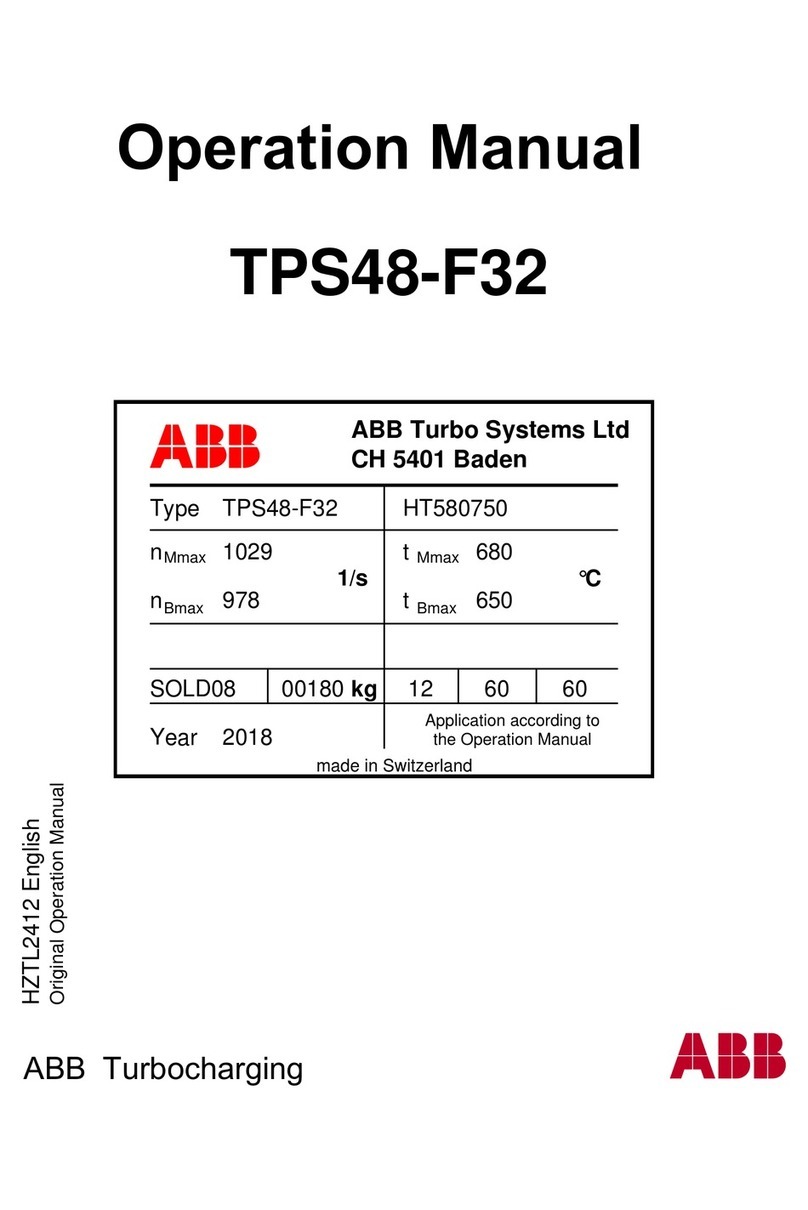
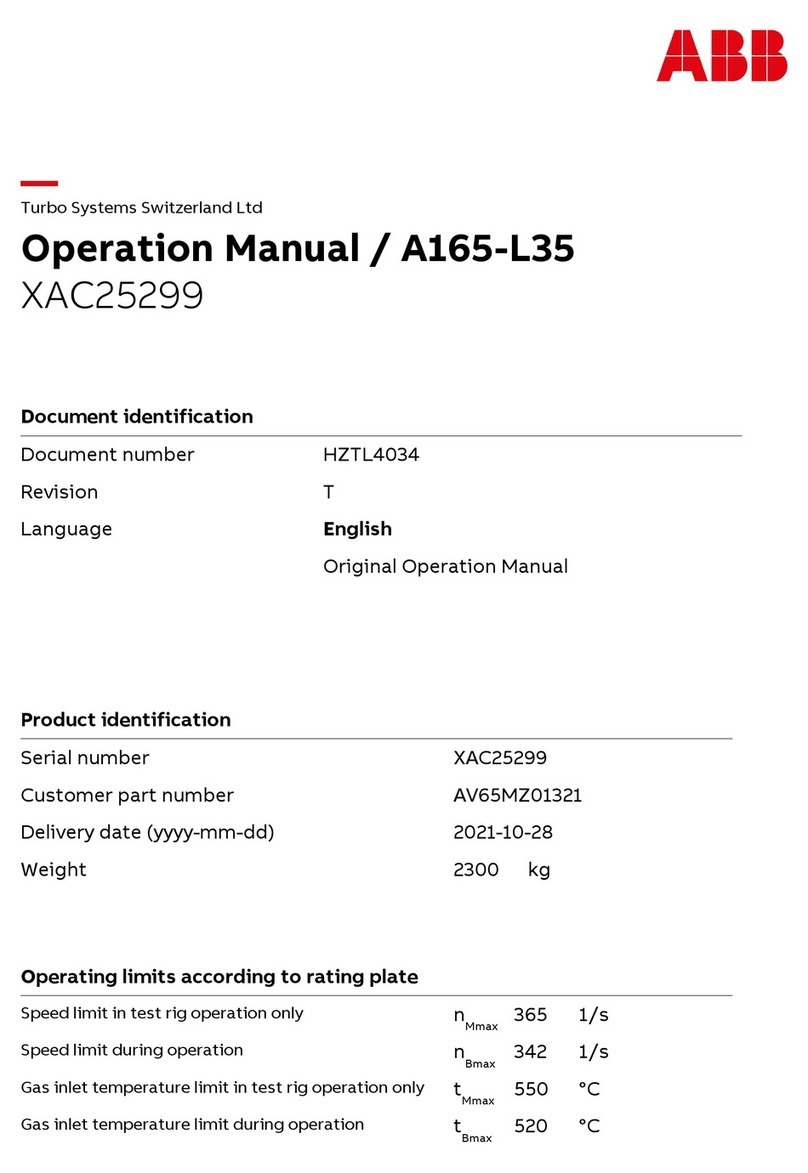
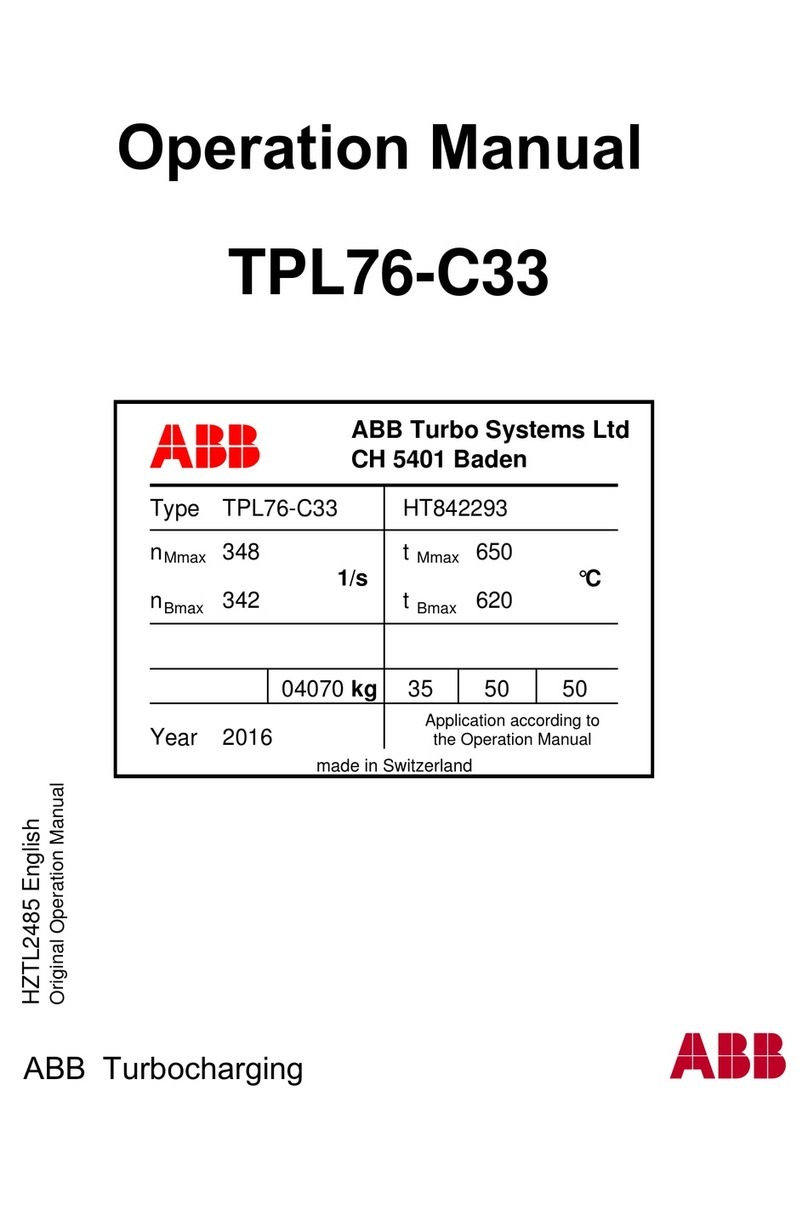
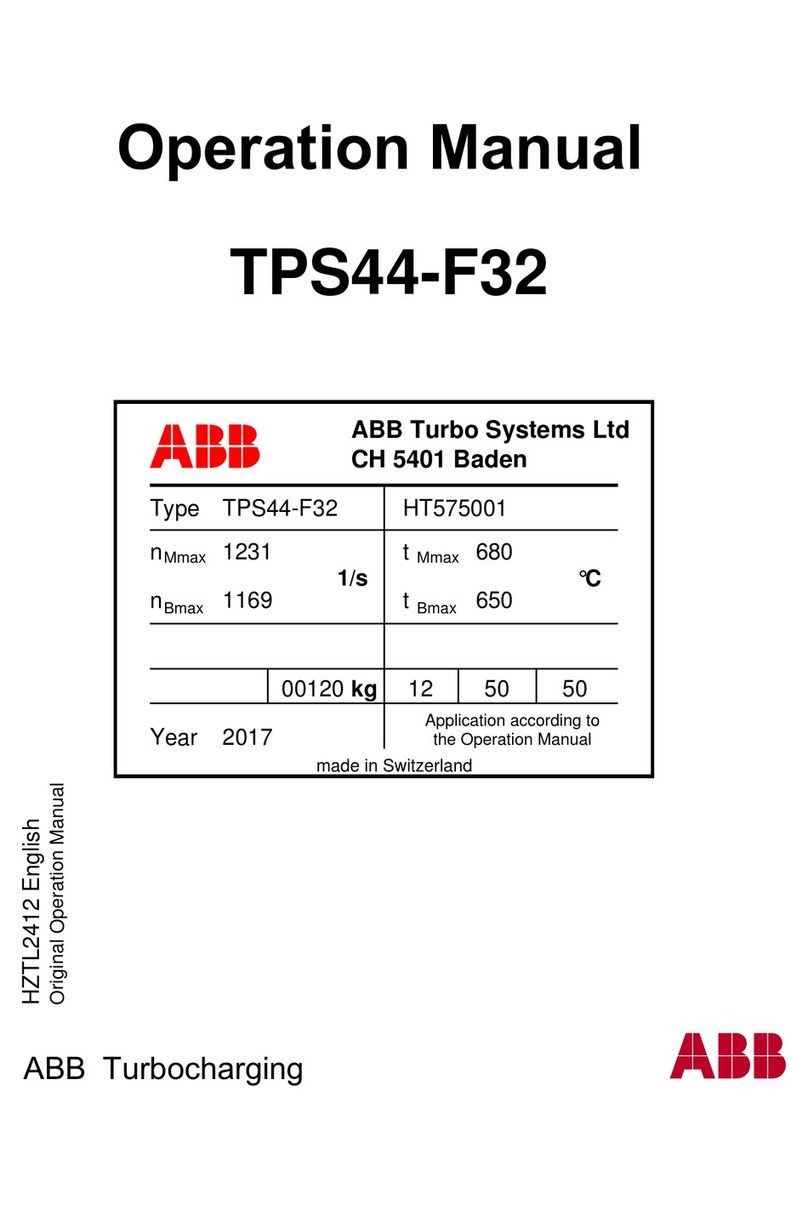
8
Note. Before proceeding any further, ensure that all switches
are set to OFF on the right hand side of the electronics unit –
see Fig. 2.3.
a) Ensure that all external electrical and plumbing connections
have been made correctly.
b) Fill the guard tubes with soda lime crystals (self-indicating).
The guard tubes clip onto the rear face of the enclosure and
breather tubes pass through grommeted holes and then to
the standard solution bottles.
c) Fill reagent and standard solution bottles and connect them
the the monitor. (See Section 8.1 for details of these
solutions.)
d) Assemble and fit the probe according to the instructions in
Sections 8.2.5 and 8.2.6.
e) Connect the electrical supply and switch on.
Note. The temperature controlled block requires up to half an
hour to reach the normal control temperature. During this
time, 'Temp. Control Error' is indicated on the display. Any
calibrations are prevented by the microprocessor during this
time.
f) Verify that there is an adequate supply of sample to the
monitor constant head unit.
g) Fit the pump platen on the peristaltic pumps (see Section
8.2.7) and switch the pumps on with the switch on the side of
the monitor. Note that the peristaltic pumps rotate, and check
that sample and reagents are being drawn into the monitor by
observing the progress of any small bubbles present in the
inlet tubes.
h) Run the monitor for at least one hour to allow the temperature
to stabilize, solutions to be pumped into the system and to
purge the air from the pipework. Check for any leaks around
the pipe connections and rectify as necessary.
i) If the monitor exhibits good stability, i.e. ±2% of reading, carry
out a calibration – see Programming Page.
j) Check the condition of the sample filter and replace it if
necessary. Ensure that new filters are fitted correctly by taking
note of the flow directions indicated on the filter bodies.
4.1 Principle of Operation
Neither bicarbonate ion content nor total carbon dioxide can
be measured directly in an untreated sample, since the probe
can respond only to free carbon dioxide gas. The bicarbonate
ions must therefore be converted to free carbon dioxide by
adjusting the pH of the sample solution to a value less than
3.4. This is effected by addition of a sulphuric acid solution to
the sample before it is presented to the probe.
The gas sensing probe in the 8237 Monitor contains a glass
pH electrode whose pH sensitive glass membrane forms a
slightly convex tip and a robust long-life reference electrode.
The two electrodes are combined into a single assembly and
are connected as a pH measuring pair through an internal
reservoir of filling solution containing bicarbonate ions.
The filling solution is 0.05M sodium bicarbonate saturated with
sodium chloride and is separated from the sample by a
gas-permeable hydrophobic membrane fitted in the tip of the
probe. Sample is caused to flow past the probe membrane,
whereupon the partial pressures of carbon dioxide gas in the
two solutions on either side of the membrane equilibrate,
transferring gas across the membrane.
At equilibrium, the concentration of carbon dioxide in the thin
film of filling solution between the probe membrane and the
glass electrode membrane equals that in the sample. The
resultant change in pH value of the thin film is measured by the
pH electrode pair which thus develops an output potential
related to the carbon dioxide concentration in the sample. Like
most ion-selective electrodes, the 8237 Probe produces an
output which is logarithmic with respect to concentration.
Under typical circumstances, with appropriate standard
solutions and calibration frequencies, accuracies better than
±5% of reading or 0.1mgl–1 whichever is the greater, can be
achieved.
4.2 General Operation – Fig. 4.1
The sequence of events is:
a) The sample enters the constant head unit from below and any
excess is allowed to overflow to drain.
The constant head unit is fitted with a float switch to signal an
'Out of Sample' condition. This switch is used by the monitor to
initiate the 'Out of Sample' alarm.
b) From the bottom of the constant head unit the sample is
drawn through the normally open ports of the solenoid valves
SV1 and SV2 by one channel of the peristaltic pump.
c) The sulfuric acid reagent is drawn through another channel of
the peristaltic pump, and is then mixed with the sample. The
tube diameters are arranged so as to obtain the correct ratio
of sample and reagent.
d) The acidified sample is allowed to react under constant
temperature conditions to release free carbon dioxide gas.
e) The sample then enters a flow-through cap at the end of the
gas sensing probe where the measurement takes place.
3SETTING UP 4LIQUID HANDLING SECTION