AED Save One D User manual

User Manual
External Semiautomatic Defibrillator
with Display
Rev.
12.2

2
QUICK USE GUIDE

3
©by A.M.I Italia s.r.l.
These instructions for use cannot, without our consent, be completely or partially
reproduced, transmitted, stored electronically or translated into another foreign
language or computer language. Infractions against this prohibition not only violate our
copyright, but also reduce our ability to provide accurate and up-to-date information to
the user and the operator of the device.
These instructions for use are subject to changes.
A.M.I Italia S.r.l.
Via Cupa Reginella, 15/A - 80010 Quarto (NA) Italy
Tel. +39 081 806 34 75 Fax +39 081 876 47 69
Printed in Italy

4
Summary
1 Introduction............................................................................................................................................... 8
1.1 Preface............................................................................................................................................... 8
1.2 Use in accordance with provisions.................................................................................................... 8
1.3 Guarantee.......................................................................................................................................... 8
1.4 Exclusion from liability....................................................................................................................... 8
1.5 Indications ......................................................................................................................................... 8
1.6 Counter indications ........................................................................................................................... 8
1.7 Version information........................................................................................................................... 9
1.8 Symbols in the manual ...................................................................................................................... 9
1.9 Manufacturer contacts...................................................................................................................... 9
2 Safety instructions................................................................................................................................... 10
2.1 Indications of DANGER .................................................................................................................... 10
2.2 Indications of CAUTION................................................................................................................... 11
2.3 Cautions for use in ECG Monitoring ................................................................................................ 12
2.4 Indications of DISPOSAL................................................................................................................. 12
3 Description of the device......................................................................................................................... 13
3.1 Device Information.......................................................................................................................... 13
3.2 Classifications .................................................................................................................................. 14
4 Description of device details ................................................................................................................... 15
4.1 General Structure of the device ...................................................................................................... 15
4.2 Keys, icons and indicators ............................................................................................................... 16
4.3 Status mini display........................................................................................................................... 16
4.4 TFT colour display............................................................................................................................ 17
4.5 Standard and optional accessories of the device............................................................................ 18
5 Parts and accessories of the Saver One D ............................................................................................... 19
5.1 Batteries Saver One D...................................................................................................................... 19
5.1.1 Non-rechargeable Li-SOCl2 battery (SAV-C0903)..................................................................... 19
5.1.2 Rechargeable Li - ion battery (SAV-C0011).............................................................................. 20
5.1.3 Suggestions for a proper maintenance of battery SAV-C0011................................................ 20
5.1.4 Inserting and removing the batteries...................................................................................... 21
5.1.5 Recharging station for rechargeable batteries........................................................................ 22
5.1.6 Structure of the battery charger ............................................................................................. 22
5.1.7 Recharge procedure ................................................................................................................ 23
5.2 PADs for defibrillation ..................................................................................................................... 24
5.2.1 Defibrillation PADs for Adults SAV-C0846 ............................................................................... 24

5
5.2.2 PADs for Children SAV-C0016.................................................................................................. 24
5.2.3 Positioning of defibrillation PADs............................................................................................ 25
5.3 2-pole ECG cable SAV-C0017........................................................................................................... 26
5.3.1 Positioning of the electrodes................................................................................................... 26
5.4 Memory Card................................................................................................................................... 27
5.5 Martel MCP7830 thermal printer (SAV-C1070)............................................................................... 28
5.5.1 Printer structure ...................................................................................................................... 28
6Saver One D selection menu.................................................................................................................... 29
6.1 Main Menu ...................................................................................................................................... 29
6.2 Settings Menu.................................................................................................................................. 30
6.3 System information Menu............................................................................................................... 31
6.3.1 Power Supply Submenu........................................................................................................... 32
6.4 Print Menu....................................................................................................................................... 33
7 Auto test.................................................................................................................................................. 34
7.1 Control LED and mini display........................................................................................................... 34
7.2 ACTIVATION test.............................................................................................................................. 35
7.3 AUTOMATIC test.............................................................................................................................. 36
7.4 POWER ON Test............................................................................................................................... 36
8 Defibrillation............................................................................................................................................ 37
8.1 Switching on the Saver One D ......................................................................................................... 37
8.2 Remove clothes ............................................................................................................................... 38
8.3 Positioning of defibrillation PADs.................................................................................................... 38
8.4 Cardiac rhythm analysis................................................................................................................... 39
8.5 Shockable rhythm............................................................................................................................ 40
8.6 Non-shockable rhythm.................................................................................................................... 41
8.7 Change of rhythm............................................................................................................................ 41
8.8 CPR................................................................................................................................................... 42
9 ECG monitoring........................................................................................................................................ 44
9.1 Activation of ECG Monitoring mode................................................................................................ 44
9.2 Description of ECG Monitoring feature........................................................................................... 46
10 Recording, printing and archiving of rescue data................................................................................ 48
10.1 Data recording................................................................................................................................. 48
10.2 Printing of rescue data .................................................................................................................... 49
10.2.1 Martel MCP7830 Printer Installation....................................................................................... 49
10.2.2 Selection of the data to be printed ......................................................................................... 50

6
10.2.3 Print execution ........................................................................................................................ 51
10.3 Data storage on PC .......................................................................................................................... 51
11 Maintenance........................................................................................................................................ 52
11.1 After each use.................................................................................................................................. 52
11.2 Ordinary maintenance..................................................................................................................... 52
11.3 Cleaning ........................................................................................................................................... 53
11.4 Preservation..................................................................................................................................... 53
11.5 Troubleshooting Guide.................................................................................................................... 54
12 Technical specifications....................................................................................................................... 55
12.1 Physical characteristics.................................................................................................................... 55
12.2 Environmental requirements .......................................................................................................... 55
12.3 Reference regulations ..................................................................................................................... 55
12.4 Technical alarms table..................................................................................................................... 56
12.5 Physiological Alarms Table (only in Monitoring Mode)................................................................... 56
12.6 Controls and indicators.................................................................................................................... 56
12.7 Data memory................................................................................................................................... 56
12.8 Defibrillator...................................................................................................................................... 57
12.9 Efficiency of delivered energy ......................................................................................................... 58
12.10 Patient analysis system................................................................................................................ 60
12.11 ECG Analysis Function ................................................................................................................. 60
12.12 ECG Monitoring ........................................................................................................................... 60
12.13 Display ......................................................................................................................................... 61
12.14 Non-rechargeable battery ........................................................................................................... 61
12.15 Rechargeable battery .................................................................................................................. 61
12.16 Internal back-up battery.............................................................................................................. 62
12.17 Rechargeable battery charger ..................................................................................................... 62
12.18 Thermal printer............................................................................................................................ 62
12.19 Defibrillation PADs....................................................................................................................... 62
12.20 ECG Cables................................................................................................................................... 63
12.21 Timing of Shock cycles................................................................................................................. 63
13 Compliance with electromagnetic emission standards....................................................................... 64
13.1 Guidelines and manufacturer's declaration - Electromagnetic emissions...................................... 64
13.2 Guidelines and manufacturer's declaration - Electromagnetic immunity ...................................... 64
13.3 Recommended separation distance between portable and mobile RF communication equipment
and Saver One device.................................................................................................................................. 66
14 Simbology ............................................................................................................................................ 67

7
15 Certifications........................................................................................................................................ 68
15.1 EC Certificate ................................................................................................................................... 68
15.2 IMQ Brand ....................................................................................................................................... 70
16 Saver One Series Defibrillator Warranty ............................................................................................. 71
17 Product registration............................................................................................................................. 72

8
1Introduction
1.1 Preface
Thank you for having chosen the defibrillator of A.M.I Italia S.r.l. model Saver One D.
So that you can correctly use the device it is necessary, before usage, to carefully read this user manual. The User
Manual of Saver One D contains the instructions for its use in compliance with its function and purpose. For a function
free of error and to achieve the right benefits, it is fundamental to respect the prescriptions indicated in this user manual,
to guarantee the safety of the patient, of the rescuer and of any third parties. This manual is an integral part of the
defibrillator and must always be kept together with the device, so that it can be easily accessible if necessary.
1.2 Use in accordance with provisions
The device Saver One D can be used exclusively if the conditions indicated in the user manual are respected. Any use
not as prescribed is considered not in accordance with the provisions and can cause damage to people or objects. In such
cases A.M.I. Italia S.r.l declines all responsibility.
1.3 Guarantee
The device Saver One D has a guarantee of 6 (six)* years.
The non-rechargeable battery Li-SOCl2(SAV-C0903) has a guarantee of 4 (four)*years in Stand-by mode (assuming a
battery activation test, daily self-tests without turning on the AED). This information refers to new batteries, fully
charged at a temperature of 20°C and humidity of 45%.
*For more information consult Chapter 16 “Saver One Series defibrillators warranty”
1.4 Exclusion from liability
The rights of liability are excluded in cases of damage to people or objects, if attributable to one of the indicated causes:
-Use of the appliance not in compliance with the provisions.
-Improper use and maintenance of the appliance.
-Use of the device and / or its accessories which show obvious or partial damage.
-Failure to comply with the instructions in the user manual concerning precautions, operation, maintenance and
repair of the appliance.
-Use of non-original accessories and/or parts not approved by the manufacturer.
-Arbitrary interventions, repairs or modifications of the device.
-Arbitrary overcoming of performance limits.
-Lack of surveillance of parts subject to wear.
1.5 Indications
The Saver One D can only be used if the patient:
-is unconscious and...
-does not breathe and...
-shows no signs of blood circulation
1.6 Counter indications
The Saver One D cannot be used if the patient:
-is in a conscious state or...
-shows normal respiration or...
-shows signs of blood circulation

9
1.7 Version information
This user manual has a version number. The version number changes every time the manual is updated for changes
made to the function of the device or to the device itself. The contents of this user manual are subject to change without
notice. The information on the version of this manual is as follows.
Version number: 12.2
Issuing date: 01/09/2020
1.8 Symbols in the manual
In this user manual there are several symbols that indicate the various precautions for use:
SYMBOL
INDICATION
DESCRIPTION
DANGER
Indicates an immediate risk to the safety of people,
which also involves death and damage to the
device or parts thereof
CAUTION
Indicates an unsafe situation or practice involving
serious personal injury and damage to the device
or parts thereof
1.9 Manufacturer contacts
You can contact our company at the following addresses:
A.M.I. Italia S.r.l.
Tel.: +39 081 806 05 74
Website: www.amiitalia.com
PRODUCTION SITE ITALY
PRODUCTION, LABORATORIES, OFFICES
Via Cupa Reginella, 15/A
80010 Quarto (NA) Italy
Phone: +39 081 806 34 75
Fax: +39 081 876 47 69
REGISTERED OFFICE
Via G. Porzio Centro Direzionale Isola G/2
80143 Napoli (NA) Italy
PRODUCTION SITE HUNGARY
PRODUCTION, LABORATORIES, OFFICES
A.M.I. International KFT
Kőzúzó u. 5/A
2000 Szentendre (Hungary)
Phone +36 26 302.210
10
2Safety instructions
For a correct use of the Saver One D defibrillator, users must be aware of the safety factors listed below.
Please read them carefully.
The Saver One D defibrillator, individually and in connection with its standard and optional (original) accessories,
complies with the safety regulations currently in force and is in compliance with the provisions of the directives on
medical products.
The appliance and its accessories are to be considered safe in the case of application according to the provisions and if
the descriptions and indications listed in this user manual are respected.
The following are the main precautions to be taken for the correct and safe use of the defibrillator, divided for easy
consultation between hazard indications, warning indications and disposal instructions.
2.1 Indications of DANGER
Use the Saver One D in accordance with the prescriptions in this user manual. Carefully read these instructions for use and in particular
the safety instructions indicated in them.
In accordance with IEC standards (section 2.4), the use of the Saver One D device or its accessories in the presence of flammable
substances (petrol or similar) or in an atmosphere enriched with oxygen or flammable gases / vapours is not allowed.
Do not recharge the Li-SOCl2 battery (SAV-C0903). Explosion risk!
Do not allow the batteries to come into contact with an open flame. Do not expose to fire.
Do not short circuit the battery terminals.
In case of leakage of liquids or strange smells from the batteries, keep them away from fire to prevent any leaked electrolytes from
catching fire.
Danger of electric shock. The device generates high voltages and dangerous levels of current.
Do not open the Saver One D, do not remove the panels and do not attempt to repair it. The Saver One D contains no components that
users can repair. For repair purposes, the Saver One D must be sent to an authorized technical service centre.
Do not apply the electrodes to the patient's chest if nitro-glycerine patches are present. Remove the patches and only then position the
electrodes. Otherwise there is a risk of causing an explosion.
Do not touch the patient and prevent third parties from coming into contact with the patient during the defibrillation shock phase. Avoid
any contact between:
parts of the patient's body
conductive liquids (such as gel, blood or solution of table salt)
metal objects in the surroundings of the patient (such as bed frame or stretching device) that represent indirect ways for the
defibrillation current
Before using the device ensure the patient’s safety, if necessary move them carefully and position them in a safe place as per the AHA /
ERC 2017 guidelines
Do not immerse any part of the Saver One D, its parts or accessories in water or other liquids.
Do not allow liquids to enter the Saver One D its parts or accessories. Avoid spilling liquid on the device and its accessories. Failure to do
so may cause damage or cause a risk of fire or electric shock. Do not sterilize the Saver One D or its accessories.

11
2.2 Indications of CAUTION
Avoid the formation of air bubbles between the skin and defibrillation PADs. The formation of air bubbles during defibrillation can cause
severe burns to the patient's epidermis. To avoid the formation of air bubbles, make sure that the electrodes fully adhere to the skin. Do not
use electrodes whose gel has dried; check the expiration date before use.
Do not delay treatment in patients with an implanted pacemaker and perform a defibrillation attempt if the patient has lost consciousness
and is not breathing or breathing normally. The Saver One D is equipped with a pacemaker detection system that allows ignoring the signal
emitted by the latter; however, with some types of pacemakers, Saver One D may discourage a defibrillation shock
During the application of the electrodes:
• Do not apply the electrodes directly to an implanted device.
• Apply the electrodes at least 2.54 cm (1 inch) from any implanted device
RF (radio frequency) interference, caused by devices such as cellular phones and two-way radios, can cause the Saver One D to
malfunction. The Saver One D must be kept at least 2 meters away from these RF devices, as indicated in the standards of
EN 61000- 4-3. Keep away from other therapeutic and diagnostic energy sources (eg diathermy, high-frequency surgery, magnetic
tomography).
Use the Saver One D only if you have achieved a BLS-D or ALS-D training course.
Before using the device, make sure that there is no obvious damage.
The infrared interface emits optically invisible radiation. The emission diode complies with IEC/EN 60825-1 Class 1 "Eye Save"
Do not use paediatric defibrillation PADS (SAV-C0016) on adult patients (older than 8 years and weighing more than 25Kg). Using
paediatric defibrillation PADS the Saver One D automatically switches to paediatric mode, reducing the maximum energy available to 50J.
Arrange the patient cables so as to reduce the possibility of wrapping or strangling the patient.
In a domestic environment, keep the defibrillator out of the reach of children and pets.
Do not apply the defibrillation electrodes directly on an implanted pacemaker to avoid any errors in the interpretation of the device and to
avoid damage to the pacemaker through the defibrillation impulse.
Disconnect high-voltage pulse-sensitive equipment from the patient, ie that is not defibrillator-proof, before delivering the shock.
CAUTION
Do not allow defibrillation electrodes to touch or come into contact with ECG electrodes, swabs, transdermal patches, etc. Failure to do
so may result in creation of electric arcs and burns to the patient during defibrillation, and even current leakage.
Position the defibrillation PADS as indicated in this user manual and indicated on the package.
Do not use defibrillation PADs if the gel has been detached from the support or is torn, split or dry.
If damage has been detected, do not operate the Saver One D under any circumstances.
Before using the device, remove metal objects from the patient's body (including necklaces or bracelets, etc.)
Do not use defibrillation PADs other than those supplied by the manufacturer. Otherwise the defibrillator may make false
interpretations.
Do not use defibrillation PADs if they are damaged, even partially.
Do not use defibrillation PADs if the expiration date has been exceeded.
When applying the ECG cable SAV-C0017 make sure it is not in contact with any conductor element. Verify that all ECG electrodes are
properly secured to the patient
Do not touch the patient or PADs during heart rhythm analysis.
Moving or transporting the patient during the cardiac rhythm analysis performed by the device can lead to an incorrect or not timely
diagnosis. During the heart rhythm analysis phase, minimize the movements. If the device is used in an ambulance in motion, stop the
vehicle and start again only after having delivered the shock.
In order to use the Saver One D, you must have completed a training course for basic or advanced cardio-pulmonary resuscitation with
the use of a defibrillator (BLS-D or ALS-D course)
Avoid the use of adult defibrillation PADs (SAV-C0846) on children (ages 1-8 years or 8-25kg).
Before applying the defibrillation PADS, if necessary, dry the patient's chest and remove unwanted hair.
Do not subject Saver One D, its accessories, its parts to falls and / or strong impacts
Do not use damaged accessories and / or parts, otherwise the device may malfunction.
Use only original accessories and / or spare parts.
Avoid excessively aggressive handling of the device of its accessories or parts in order to avoid possible damage. Inspect the entire
system periodically.
Carry out the sanitation operations of the device in compliance with the standards indicated in paragraph 10.3 and always make sure that
the device is switched off with the battery removed and PADs disconnected.
Defibrillation PADs are disposable, to be used only on one patient. Do not reuse defibrillation PADs; discard after use and replace with a
new pair.
Defibrillation PADs are not sterile or sterilizable.
Recharge the rechargeable Li-ion battery (SAV-C0011) at least once every 4 months ensure its perfect function and extend its life.
The Li-ion rechargeable batteries ACC model (SAV-C0011) must be charged using only the (SAV-C0012) battery charger from A.M.I.
Italia S.r.l. otherwise the batteries could be damaged
Remove the batteries from the device only if it has been turned off for at least 5 seconds. Otherwise the device and the battery can be
seriously damaged.
The Saver One D, its parts and accessories are not sterile or sterilizable
Do not expose the Saver One D, its parts or accessories to direct light or high temperatures
The Battery Charger (SAV-C0012) must only be used with the Meanwell power supply model GS40A15-P1J (SAV-C0013) supplied by
A.M.I. Italia S.r.l. The use of different power supplies could compromise the correct functioning of the battery charger and damage the
ACC rechargeable batteries (SAV-C0011)
In order to safeguard the battery life (SAV-C0903) and guarantee automatic daily tests, after installing it, it is advisable to not remove
the battery (SAV-C0903) unless it is to be replaced. The removal of the battery and the subsequent insertion involves a complete test of
the AED which considerably consumes its capacity. Furthermore, if the battery is not properly attached it could be damaged.

12
2.3 Cautions for use in ECG Monitoring
The monitoring mode based on the use of the screen, for the purpose of identifying an ECG rhythm, represents an important aid for the
specific use of the device itself, i.e. the detection of a shockable rhythm for the eventual subsequent decision to release of a therapeutic
shock. The monitoring mode is intended for those environments or rescue conditions where experienced operators, or under the specialized
medical direction, may have the benefit of evaluating patients with a high risk of a cardiac event that can be life threatening. By switching
the defibrillator operation from the analysis mode to the monitoring mode, the device continues to analyse the patient's ECG and, if a
potentially shockable rhythm is detected, the operator can return to defibrillation mode and prepare to shock. In any case, do not use the
device in "ECG Monitoring" mode in environments such as operating rooms or intensive care units and consequently with medical
equipment typical of such environments (such as for example an electrosurgical unit). Moreover, for the intended use, the device does not
guarantee completely suitable display performance in the presence of patients with pacemakers.
Use the device only with accessories (patient cables, electrodes, adhesive clips) supplied by AMI Italia following the instructions indicated
in this manual for their application.
Pay attention that the conductive parts of the electrodes do not come into contact with other conductive parts, including the floor.
As a precaution, if there is a defibrillator connected to the patient with whom a defibrillation shock can be delivered, avoid touching the
patient while undergoing ECG Monitoring and, to ensure the necessary protection, use only accessories (patient cables, electrodes,
adhesive clips) supplied by AMI Italia and listed in this manual.
In the presence of patients with pacemakers, the calculation of heart rate could count pacemaker pulses even in the event of cardiac arrest
or some arrhythmias. In this case, do not rely completely on alarms related to the counting of beats. Monitor patients with pacemakers and
follow the instructions in this manual regarding pacemaker pulse rejection capabilities of this device.
In the presence of patients with pacemakers, the parameter values presented by the device may not be sufficiently accurate. In this case,
these should not be used to draw medical conclusions.
The device is able to recognize and manage T waves appropriately up to a maximum width of 1 mV.
To monitor whether the electrodes have been applied to the patient, the device injects a sinusoidal current of Ipp = 0.5mA and f = 25.2
KHz.
The frequency of the QRS complexes is calculated by making the arithmetic average over 3 consecutive intervals (4 QRS complexes) and
the value shown on the display is updated every second.
Delays in determining the alarm conditions relating to the monitoring mode are contained within 5 seconds, except for the LOW
FREQUENCY and ASYSTOLE alarms for which the signalling delay remains within 10 sec. In this case, in fact, in the lower limit
conditions (30bpm), there are 2 sec between two consecutive beats, and since the QRS detection algorithm requires 4 complexes, the time
required to identify an LF (LOW Frequency) alarm condition is greater than 6 seconds. For ASYSTOLE signalling, having to exclude first
that it is not an LF condition and then confirm that it is ASYSTOLE, the signalling time is greater than the previous one by about 2
seconds (about 8 seconds).
The device takes less than 3 seconds to switch the indication of 80 bpm to 120 bpm and vice versa.
The device takes less than 3 seconds to switch the indication of 80 bpm to 40 bpm and vice versa.
For the two waveforms of fast ventricular tachycardia: 195 bpm @ Vpp = 2mV, 1mV, 4mV and 206 bpm @ Vpp = 1mV, 0.5mV, 2mV,
the device signals the alarm condition within 5 seconds.
In the event of an alarm, the sound emitted is composed of at least 4 different frequencies so that it can be heard even by people who have
partially impaired hearing. Simultaneously, icons and descriptions of the detected alarm status are shown on the display.
The device guarantees 35 hours of continuous monitoring with a new fully charged battery.
2.4 Indications of DISPOSAL
The Saver One D, its parts and accessories must not be disposed of with other household waste within the European community. To
prevent possible damage to the environment or human health caused by incorrect waste disposal, recycle this product responsibly also to
promote sustainable use of resources. To dispose of the used product, use the appropriate waste collection services or return it to the
local distributor. In this way it will be possible to recycle safely for the environment.

13
3Description of the device
3.1 Device Information
The Saver One D is called AED or Automatic External Defibrillator equipped with TFT display and mini LCD.
The device was designed to be used by lay personnel as well as by healthcare personnel who have duly achieved and
passed a BLSD course according to international guidelines.
Designed to automatically detect and analyse the victim's heart rhythm, it is able to deliver one or more defibrillation
shocks if ventricular fibrillation or ventricular tachycardia (monomorphic or polymorphic with beat> 180) is detected.
The energy is supplied by an exponential truncated biphasic electrical shock (B.T.E.) able to adapt to the patient's
thoracic impedance.
The Saver One D is available in two versions:
Saver One D 200J (SVD-B0004) –Maximum deliverable energy of 200J
Saver One D 360J (SVD-B0005) –Maximum deliverable energy of 360J
It can be used with two types of batteries:
Non-rechargeable battery Li-SOCl2 (SAV-C0903), which requires no maintenance, is guaranteed to operate
in standby mode for 4 years or carry out a high number of shocks
ACC Rechargeable battery Li-ion (SAV-C0011), recommended for those who use the defibrillator
intensively
The device is equipped with a large 5.7-inch LCD colour display that allows you to view all information relating to the
treatment and its functional status. Furthermore, the Saver One D is equipped with a mode that allows the patient's ECG
monitoring to be performed using a special 2-pole ECG cable (SAV-C0017) with detection of 1 lead (II) or directly
from the Pads.
The device allows the data to be recorded on an SD Memory Card so that they can be re-displayed on a PC or printed
directly on the Martel MCP7830 printer (optional function). During the non-use phase the device, if installed, the
battery carries out daily self-tests to verify its functional condition, in order to guarantee its prompt use in the moment
of need. On the keyboard of the device there is a mini LCD display and a two-colour LED (red / green) through which it
is possible to see the outcome of the functional tests and to know the functional status of the device even if switched off
(stand-by mode).

14
3.2 Classifications
The Saver One D defibrillator is classified as follows:
Code UMDNS
11132
Code GMDN
47910
Code CND
Z12030501
Directory number RDM
238278 / 1535710
Code CIVAB
DEF01
Class of belonging according to directive 2007/47/CE
IIb
Type of protection against electric shock
Internally powered
Type of patient isolation
BF
CF (only for ECG cables)
Degree of protection against penetration of liquids
IPx4
Degree of protection against dust penetration
IP5x
Degree of safety in the presence of a flammable anaesthetic
mixture with air, oxygen or nitrous oxide
Not protected
Sterilization or disinfection method suggested by the supplier
See Paragraph 11.3
Mode of operation
Continuous operation
15
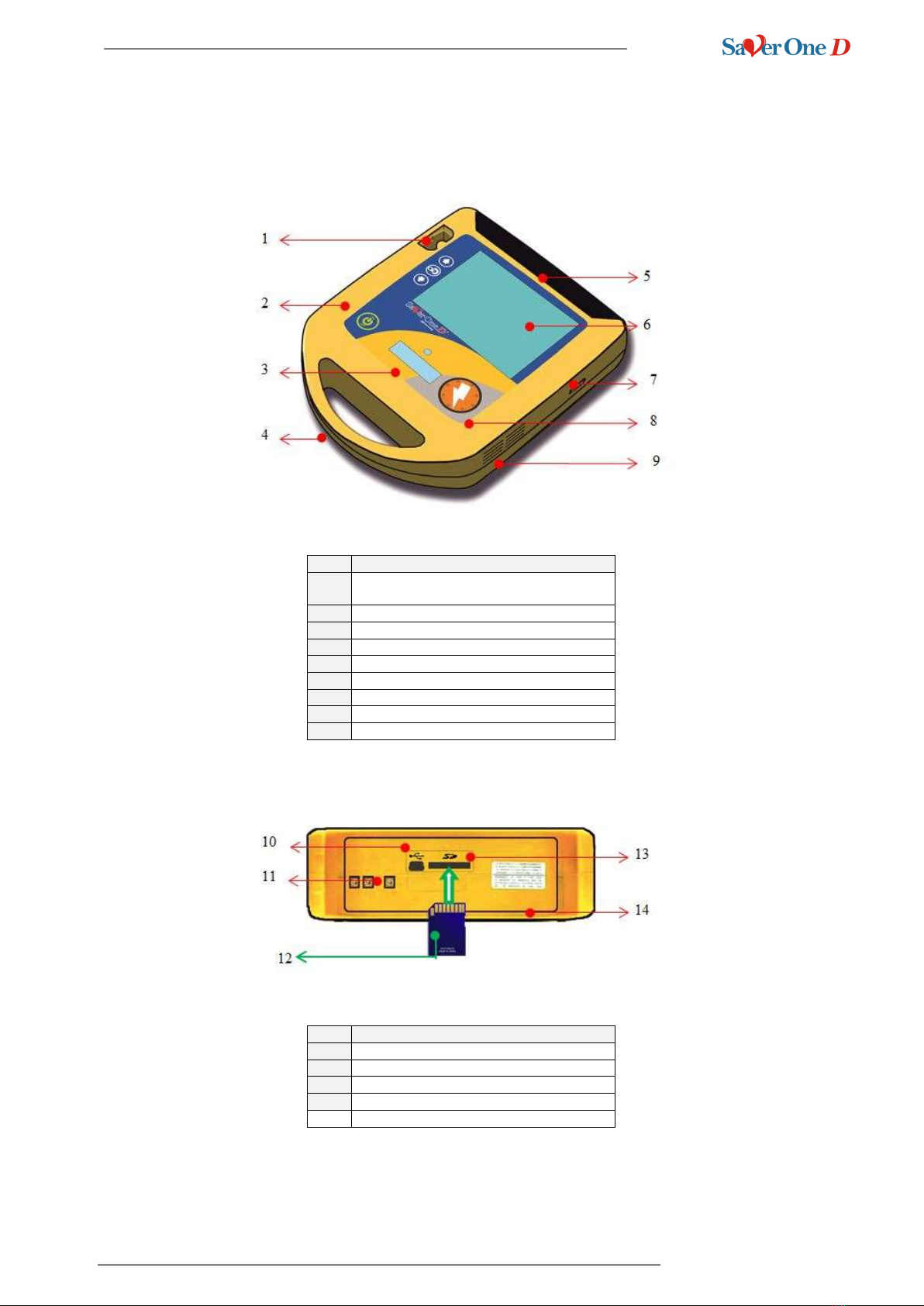
4Description of device details
4.1 General Structure of the device
Nr.
Description
1
Compartment for PADS connector or ECG
cable
2
Microphone for environmental recordings
3
Status mini display
4
Carrying handle
5
Battery
6
TFT colour display
7
IrDA port (service only)
8
Keyboard with buttons
9
Loudspeaker
Nr.
Description
10
USB port
11
Battery contact tabs
12
SD Memory Card insertion
13
SD Memory Card port
14
Gasket
Image 2
Image 1
16
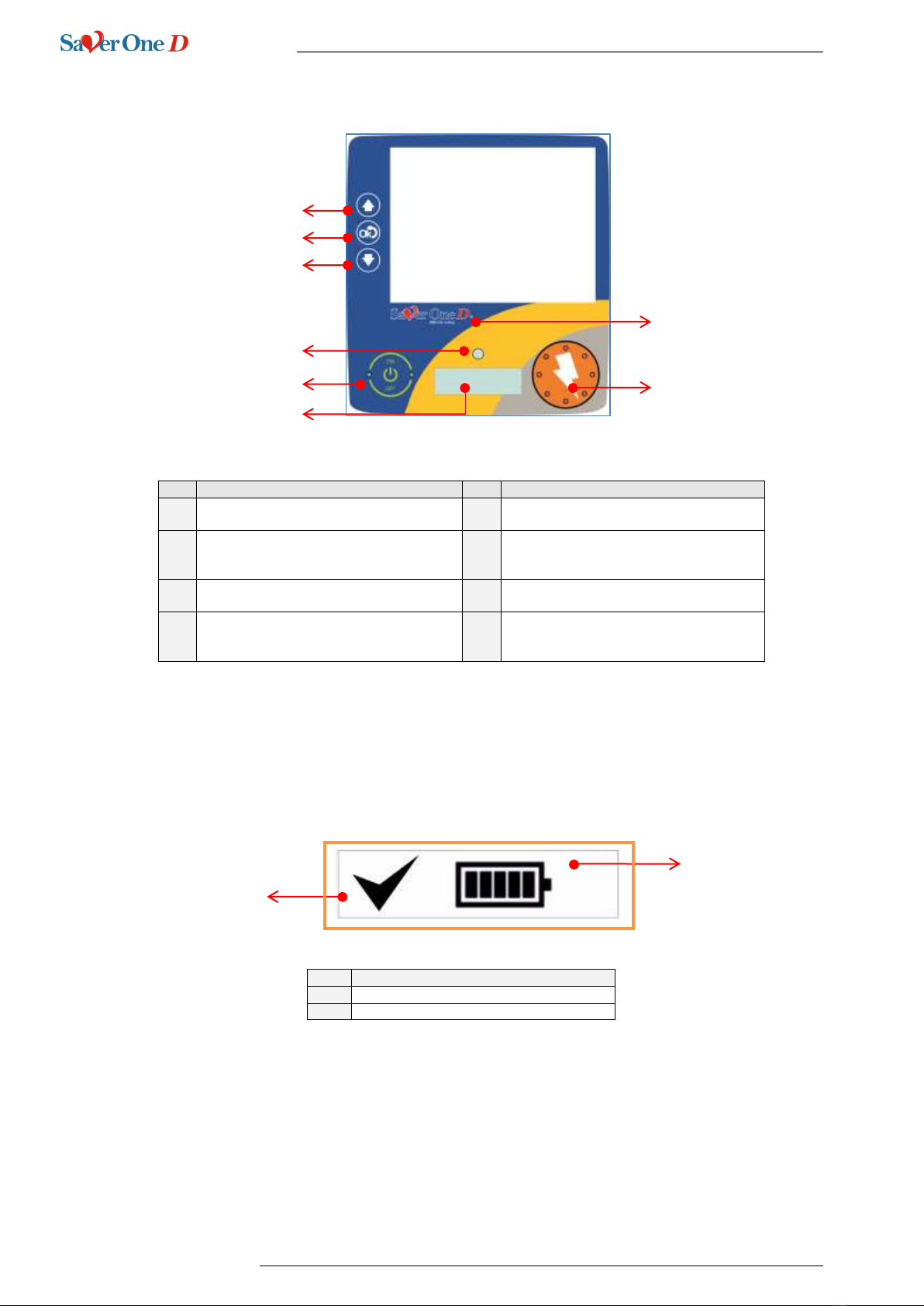
4.2 Keys, icons and indicators
Nr.
Function
Nr.
Function
1
Navigation key UP
Allows you to scroll up the menu
5
ON / OFF button
Allows you to switch the device on or off
2
Navigation key ENTER
Allows you to enter the menu and confirm
the selection you made
6
Status mini display
It allows you to check the functional status
of the device
3
Navigation key DOWN
Allows you to scroll down the menu
7
Product logo
Indicates the model of the device
4
Control LEDs
Luminous LED (red / green) allows you to
check the functional status of the device
8
Shock button
Equipped with luminous LEDs it allows to
deliver a defibrillation shock if indicated
4.3 Status mini display
The mini display is designed to inform the user about the functional status of the device and its battery even when the
device is switched off (stand-by mode).
Nr.
Description
1
Functional status of the device
2
Remaining battery level
Image 3
1
4
3
5
6
8
2
1
2
7
17
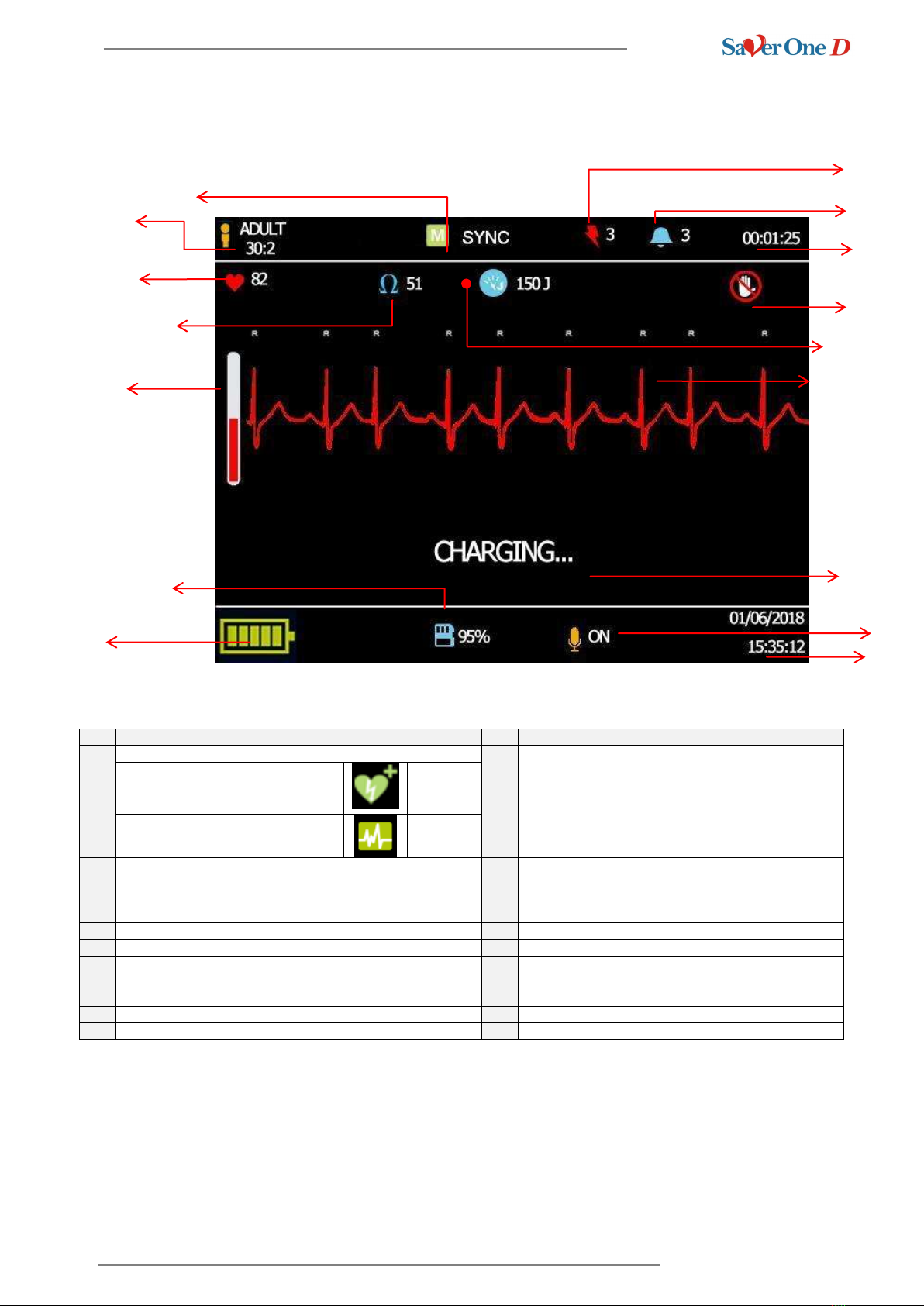
4.4 TFT colour display
Nr.
Description
Nr.
Description
1
Indicates the OPERATIVE MODE
9
Indicates the number of VFs and / or VTs detected by
the device
DAE:
Semiautomatic defibrillation
AED
MODE
MONITORING:
ECG monitoring
ECG
2
Indicates the type of patient to be treated and
Ratio Compressions/Insufflations:
Adult 30:2
Paediatric 30:2 (requires children pads)
10
Energy in charge and subsequently delivered
3
Indicates the patient's heart rate
11
Indicates not to touch the patient in certain operations
4
Indicates the patient's thoracic impedance detected
12
Energy in charge and subsequently delivered
5
Progressive charging bar
13
ECG track of the patient
6
Indicates the remaining level of the SD Memory Card
14
Text command that instructs the operation to be
performed
7
Indicates the remaining battery level
15
Indicates whether the recording microphone is active
8
Indicates the number of shocks made
16
Indicates current date and time
2
3
4
5
6
7
10
13
14
15
16
Image4
1
12
11
9
8
18
4.5 Standard and optional accessories of the device
The Saver One D defibrillator comes with the following standard accessories:
Code
Image
Quantity
Description
SVD-B0004
1 Unit
(Version 200J or 360J)
Saver One D 200J
SVD-B0005
Saver One D 360J
SAV-C0846
1 Unit
Adult Pads
SAV-C0903
1 Unit
Non-rechargeable
Li-SOCl2battery
SAV-C1005-HU
1 Unit
User guide
SAV-C0916
1 Unit
Carrying case
The following are the optional Saver One D accessories that can be purchased separately:
Code
Image
Quantity
Description
SAV-C0011
1 Unit
ACC Rechargeable Li ion battery
SAV-C0012
1 Unit
CBACCS1 Charger
SAV-C0013
1 Unit
GS40A15-P1J Power supply
SAV-C0014
1Unit
(Contains 3 units)
N.01 CBACCS1 Charger
N.01 GS40A15-P1J Power supply
N.01 Power supply cable
SAV-C0016
1 Unit
Children Pads
SAV-C0017
1 Unit
2-way ECG cable
SAV-C0019
1 Unit
CD-ROM Saver View Express
SAV-C0906
1 Unit
SD Card
SAV-C1070
1 Unit
Thermal printer MARTEL MCP7830
SAV-C0027
1 Unit
Memory Card reader for PC

19
5Parts and accessories of the Saver One D
5.1 Batteries Saver One D
The Saver One D defibrillator can work with two different types of batteries:
(SAV-C0903) Non-rechargeable Li-SOCl2 battery
(SAV-C0011) ACC Rechargeable Li ion battery
For AED models Saver One D and Saver One P, considering the higher consumption due to the presence of the TFT
display, AMI ITALIA recommends the use of the rechargeable battery SAV-C0011 (combined to the charging station
SAV-C0014) rather than the disposable battery SAV-C0903.
5.1.1 Non-rechargeable Li-SOCl2 battery (SAV-C0903)
The non-rechargeable battery with Li-SOCl2technology (SAV-C0903) is supplied fully charged and ready for use. The
Li-SOCl2 non-rechargeable battery has been designed to have a long battery life and no maintenance whatsoever.
The non-rechargeable battery of the Saver One D in Standby mode is guaranteed for 4 (four) years*1assuming a battery
activation test, daily self-tests without turning on the AED. The Li-SOCl2non-rechargeable battery (SAV-C0903) is
able to carry out a large number of shocks which vary according to the version:
Saver One D Standard 200J 250 complete rescue cycles (shocks at 200J. and CPR)*1
Saver One D Power 360J 160 complete rescue cycles (shocks at 360J. and CPR)*1
*1New and fully charged battery, constant temperature at 20°C and relative humidity without condensation 45%
If the remaining battery level is low, the Saver One D informs the user via audio and visual messages.
The Saver One D will give a low battery warning when the level is ≤5% (WARNING) and a very low battery
warning when the level is ≤ 1% (ALARM)
WARNING: Remaining capacity level of Battery equal or less than 5%.
This notice will only be provided in Operating mode as indicated in paragraph 5.1.
With a 5% battery the Saver One D allows to shock about 14 shocks or 40 days of stand-by*2
ALARM: Remaining capacity level of Battery at ≤ 1%
This warning will be provided both in Stand-by and in operating mode, as indicated in the paragraph
With a battery at ≤ 1% the Saver One D carries out about 7 shocks or 20 days of stand-by*2
In this condition the use of the device is not recommended.
*2, Constant temperature at 20°C and relative humidity without condensation 45%
!!ATTENTION!!
In order to protect the battery life (SAV-C0903) and guarantee automatic daily tests, after installing it, it is
advisable not to remove the battery (SAV-C0903) unless it is to be replaced. The removal of the battery and the
subsequent insertion involves a complete test of the AED which considerably consumes its capacity.
Furthermore, if the battery is not properly attached it could be damaged.
Image 5

20
5.1.2 Rechargeable Li - ion battery (SAV-C0011)
The rechargeable battery with Li-ion technology (SAV-C0011) of the Saver One D is suitable for those who use the
defibrillator intensively. Being rechargeable, it allows operators to reduce management costs and guarantee a greater
number of interventions.
The ACC rechargeable battery of the Saver One D can be recharged using only the dedicated charger (SAV-C0012)
with relative accessories supplied by A.M.I. Italia S.r.l. The battery allows you to carry out a high number of shocks
which varies according to the version of the Saver One D in your possession:
Saver One D Standard 200J typically 200 continuous shocks *1
Saver One D Power 360J typically 110 continuous shocks *1
*1 New and fully charged battery, constant temperature at 20°C and relative humidity without condensation 45%
If the remaining battery level is low, the Saver One D informs the user via audio and visual messages.
The Saver One D will give a low battery warning when the level is ≤5% (WARNING) and a very low battery
warning when the level is ≤ 1% (ALARM)
WARNING: Remaining capacity level of Battery equal or less than 5%.
This notice will only be provided in Operating mode as indicated in paragraph 5.1.
With a 5% battery the Saver One D allows to shock about 14 shocks or 40 days of stand-by*2
ALARM: Remaining capacity level of Battery at ≤ 1%
This warning will be provided both in Stand-by and in operating mode, as indicated in paragraph 5.1
With a battery at ≤ 1% the Saver One D carries out about 7 shocks/20 days of stand-by*2
In this condition the use of the device is not recommended.
*2, Constant temperature at 20°C and relative humidity without condensation 45%
It is advisable to replace these batteries every 2 years or after having made a number of recharges greater than 300 (the
event that occurs first).
5.1.3 Suggestions for a proper maintenance of battery SAV-C0011
A.M.I Italia recommend that batteries SAV-C0011 left in a “storage stage” to be fully recharged at least every 4
months from the receipt of the goods and to be recharged regularly every 4 months when attached to the device "ready
to use" , to avoid completely discharging it and to maintain maximum life expectancy of the battery. The battery pack
technology and the modules offered are to ensure a long lasting duration but they require a correct maintenance; failure
to follow these requirements will result in an early deterioration of the battery, which will not be covered by warranty.
For warranty replacement consideration, batteries are to be returned to the original supplying distributors/dealer.
Image 6
This manual suits for next models
1
Table of contents
Other AED Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Welch Allyn
Welch Allyn Spot Vital Signs LXi Directions for use
GETINGE GROUP
GETINGE GROUP Arjo Huntleigh Citadel Plus ENT-ACC101 Instructions for use

Gima
Gima 34020 Use and maintenance book

Gima
Gima KS-H1N manual

Bioventus
Bioventus EXOGEN Quick instruction guide

MGE UPS Systems
MGE UPS Systems SAM Operating and maintenance manual

Accu-Chek
Accu-Chek FlexLink instructions

Magene
Magene H303 manual
BMC
BMC H-80 Series Quick operation manual

Top shelf Orthopedics
Top shelf Orthopedics Wrist/Thumb Lace-Up Supports Instructions for use
bort medical
bort medical Select StabiloGen Instructions for use

Elektron Technology
Elektron Technology HENSON 8000 user manual