AED HeartPlus NT-180 Guide

i
HeartPlus
™
User Manual & Warranty Registration
©2016 NANOOMTECH CO., LTD All rights are reserved. It is prohibited to reproduce or
duplicate, in any form, this manual or any part thereof without permission of NANOOMTECH
CO., LTD.
This user manual may be changed without prior notice to users for functional improvements. If
more than two (2) years have elapsed since the revision date (listed on the bottom of this
page), please contact NANOOMTECH CO., LTD or your local distributor to determine whether
additional product information updates are available.
HeartPlus™is a trademark of NANOOMTECH CO., LTD
EM0101-2 (1810-REV06)
Publisher: NANOOMTECH CO., LTD.
This issue date for the HeartPlus™User Guide, Rev. 6, is Oct 2018.
Device manufacturer
NANOOMTECH CO.,LTD.
Address: 57, Cheomdan venture so-ro, Bukgu, Gwangju, 61003, Rep. of Korea.
Authorized EU Representative
CMC Medical Devices & Drugs S.L.
C/ Horacio Lengo Nº 18, CP 29006, Málaga, Spain
Tel: +34951214054 / Fax: +34952330100 / E-Mail: info@cmcmedicaldevices.com

ii
Intentionally Blank

iii
Table of Contents
HeartPlus™-User Manual & Warranty Registration
ABSTRACT ..............................................................................................................1
SIMPLIFIED CPR....................................................................................................2
SECTION I - PRODUCT INTRODUCTION..........................................3
CONTRAINDICATIONS...........................................................................................4
PRODUCT INFORMATION.....................................................................................5
PRODUCT SPECIFICATION AND FUNCTION........................................................6
SET CONFIGURATION............................................................................................9
PRODUCT CHARACTERISTICS............................................................................ 11
DISPLAY DETAILS ................................................................................................12
NAME AND FUNCTION OF COMPONENTS..................................................... 14
SECTION II - PRODUCT USE ............................................................ 19
SIMPLE GUIDE FOR USE.....................................................................................20
HOW TO INSTALL A BATTERY PACK .................................................................21
HOW TO INSTALL ELECTRODE PADS................................................................ 23
DETAILED INSTRUCTIONS (ADULT) .................................................................. 24
DETAILED INSTRUCTIONS (PEDIATRIC)............................................................29

iv
BATTERY CHECK..................................................................................................34
PC COMMUNICATION....................................................................................... 35
SECTION III - GUIDELINES ............................................................... 37
CHECK LIST -PRIOR TO USE ............................................................................38
POST-USE PRODUCT MANAGEMENT .............................................................. 41
STORAGE GUIDELINES........................................................................................43
CAUTIONS FOR BATTERY PACK......................................................................... 44
GLOSSARY OF SYMBOLS ................................................................................... 46
SECTION IV - APPENDIX.................................................................. 49
DEFIBRILLATION WAVEFORM............................................................................ 50
CHARACTERISTIC OF ECG ANALYSIS ................................................................ 52
ELECTROMAGNETIC COMPATIBILITY (EMC)..................................................... 53
VOICE PROMPTS ................................................................................................56
DIAGNOSTICS &TROUBLESHOOTING............................................................. 57
SERVICE &WARRANTY .................................................................................... 59

1
Abstract
HeartPlus™(NT-180) is an Automated External defibrillator which restores
a normal heartbeat in patients with SCA (sudden cardiac arrest).
HeartPlus™(NT-180) is an emergency medical device.
Before using this product, please read carefully the safety precautions and
instructions for proper usage.
HeartPlus™(NT-180) is a medical device which must be tracked and
monitored to ensure optimal performance and operability.
If the HeartPlus™(NT-180) is sold, donated, lost, stolen, exported, or
destroyed, please notify NANOOMTECH CO., LTD or your local distributor.
The following visuals are used in this manual to help encourage safe and
proper usage and maintenance of the product.
Indicates possibility of a potentially dangerous
situation resulting in death or severe injury in the
case where instructions are not followed.
Indicates possibility of injury or product damage
that results in the breach of warranty agreement.
Indicates current manufacturer policy for proper
use of the product and accessories.

2
Simplified CPR
OHCA Chains of Survival
1
The recommended compression rate is at least 100 compressions per
minute.
Be sure to achieve the full compression depth of at least 5cm
(2 inches) for adult patients.
For children, be sure to achieve the full compression depth, which
should be at least 1/3 of chest anteroposterior diameter of
approximately 4cm (1.5inch) for infants and approx. 5cm (2inch) for the
child.
Achieve enough compression speed and depth, allow the chest to
recoil completely after each compression, minimize interruptions in
chest compression, and refrain from excessive artificial respiration.
Perform the chest compression before one-man rescuer tries artificial
respiration. (Perform in order of chest compression 30 times, then
followed by artificial respiration 2 times.)
1
Source: 2015 America Heart Association (AHA) guideline for CPR and cardiovascular first aid.

3
Section I
Product Introduction

Section I -Product Introduction
4
Product Introduction
Product Description
HeartPlus™is an AED (Automated External Defibrillator) which provides
defibrillation treatment for quick resuscitation of an SCA (Sudden Cardiac
Arrest) patient. This product is a portable one operating on batteries. It is
reasonably light and can be operated even by the user who receives
minimal or no education/training on the use of the product through voice
commands that guides the user through the operation sequence.
SCA (Sudden Cardiac Arrest) is the cessation of normal circulation of the
blood due to the failure of the heart. SCA can occur to anyone at anytime
and anywhere. SCA patients do not show any warning signs or symptoms.
Some individuals are at a higher risk of suffering from SCA than others
due to hereditary or other reasons.
When to Use
Patient does not respond to voice.
Patient has no reaction even when shaken.
Patient does not breathe normally.
If you suspect that the patient shows the above symptoms, follow the
voice commands after turning on this product at every stage (attaching
electrode pad, shock or no shock, CPR).
Contraindications
None known.
Product User
HeartPlus™should be used by a person who has been educated on the
use of AED (Automated External Defibrillator) and CPR (Cardiopulmonary
Resuscitation).
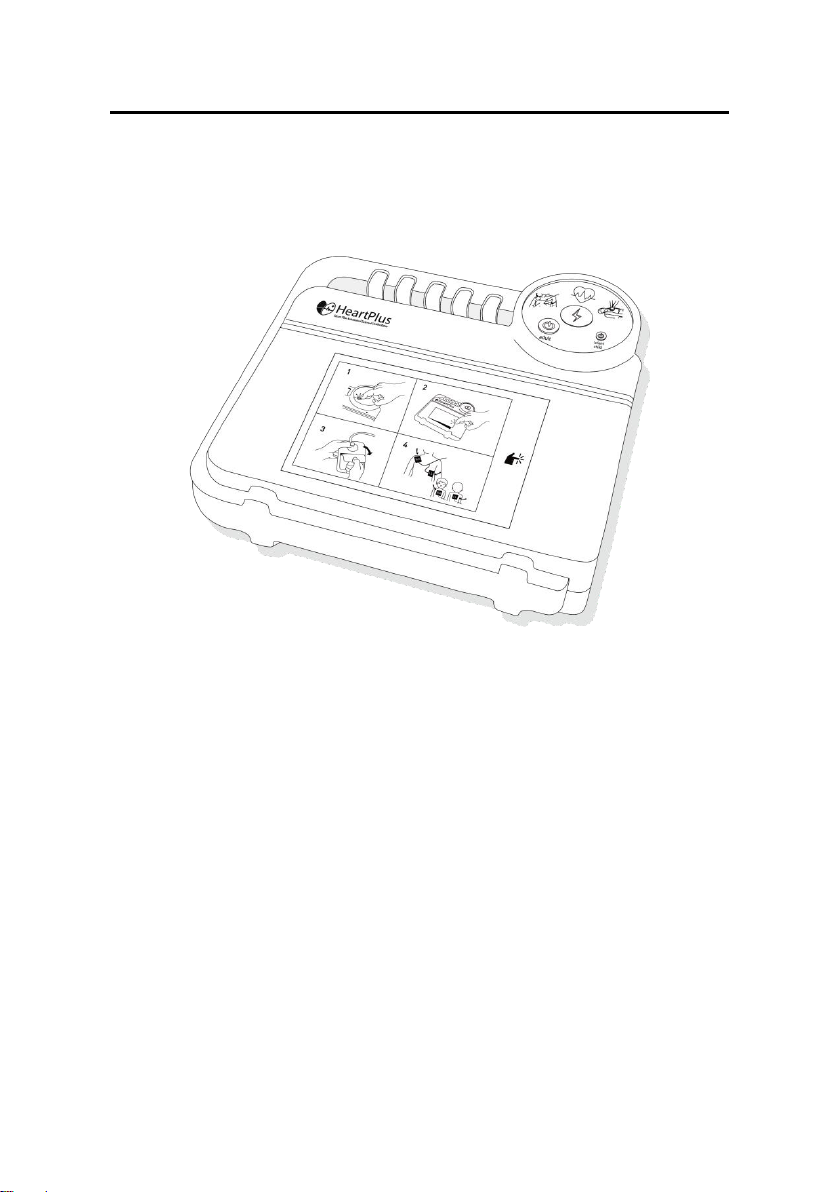
Section I- Product Introduction
5
Product Information
Product Name: AED
Brand Name: HeartPlus™
Model Name: NT-180, NT-180Y, NT-180B, NT-180G
Product Appearance:
Size: 293mm(W) X 291mm(L) X 71mm(H)
Weight: 1.9kg (Incl. cartridge)

Section I -Product Introduction
6
Product Specification and Function
Defibrillator
Discharge Current Waveform: BTE (Biphasic Truncated
Exponential)
Discharge Time: Automatically adjusted energy for
patient impedance
Output Energy:
-180J (for adult, load impedance of 50)
-50J (for infant, load impedance of 50)
Patient Insulation: BF Type
Charging period: Less than 13 seconds after pads
attachment.
Operation Mode: AED (automated external
defibrillator), which can analyze ventricular fibrillation
Change Method to Pediatric Mode: Power on device
in Pediatric Mode (small yellow button)
ECG
Induction Method of ECG: LEADⅡ
All-in-one Type
Cartridge
(Pre-connected
Battery And
Electrode Pads)
Basic
Specification
Composition: Disposable Electrode
pads, Battery
Frequency of Use: One time
Packing Method: Sealing Packaging
Size: 150(W) X 110(L) X 33(H)mm
Warranty: 2 years (Sealed storage at
room temperature)
Electrode
Pads
Frequency of Use : Single Use
Size: 125mm X 95mm
Area: 76.63㎠(PE Foam, Hydrogel)
Wire Length: 1.2m
Attachment point: Showed in picture on

Section I- Product Introduction
7
Electrode pads.
Composition: Composed of two
electrodes
Battery
Type: Non-rechargeable Lithium-ion
battery
-DC 21V (1,400mAh)
-DC 9V (1,400mAh)
Detachable
Type Cartridge
(Separate
Battery and
Electrode Pads)
Type: Non-chargeable Lithium-ion battery
-DC 21V (1,400mAh)
-DC 9V (1,400mAh)
The number of defibrillation shock can be marked on
the cartridge.
Only a detachable type electrode pads can be used.
Size: 150(W) X 110(L) X 33(H)mm
Warranty: About five (5) years (Sealed storage at
room temperature)
Detachable
Type Electrode
Pads
Disposable
Area: 86.44cm2(Hydro Gel)
Wire length: 2m±20%
Attachment point shown in pictures on electrode pads.
Composed of two (2) electrodes attached to pads
Warranty: About two (2) Years
Alarm and
Voice Prompts
Voice prompts for operational use for both instances
where ventricular defibrillation is required or should be
bypassed.
Voice prompts of all procedures for providing first aid
to the patient.
Voice prompts for the self-test result.

Section I -Product Introduction
8
Configuration
Main unit
Cartridge (all-in-one type)
Cartridge (detachable type)
Electrode pads (detachable type)
Protection
Function
In case the electrode pads are not attached to the
patient, device cannot administer defibrillation shock.
If defibrillation shock is not executed by way of
pressing the flashing “shock button” within 20
seconds, the charged defibrillation shock energy is
automatically discharged internally.
Over-discharge protection circuit for proper
discharging of defibrillation shock energy.
Safety control circuit to prevent the malfunction of
the electric shock waveform control circuit.
Storage And
Management
Of
Data/Event
Stored content:
-Electrocardiogram.
-The number of defibrillation shocks
-Surrounding sound during the operation of the
product.
-Self-test time and result
-Time and date of product use.
Storage time:
-5 second during analysis only, 1,000 ECGs
(analysis only)
-Surrounding sound: Max. 15 minutes
-Self-test result: Max. 3,000 cases
Data communication: USB to PC (Type B to Type A)
Data storage method:
-embedded memory

Section I- Product Introduction
9
Set Configuration
The product is composed of a main body, a cartridge, and other
components (a towel, a USB cable, etc.)
In case of cartridges, there is All-in-one type of cartridges where an
electrode pads and a battery are not separated and detachable type of
cartridge where a battery and electrode pads are separated.
HeartPlus™Main unit

Section I -Product Introduction
10
Set Configuration (options)
Cartridge (All-in-one type)
electrode pads
Consumables for a detachable type
Cartridge (detachable type)
electrode pads (detachable type)
Other consumables
Towel
USB cable(Option)

Section I- Product Introduction
11
Product Characteristics
User-friendly device management (all-in-one type/detachable type)
Decides if it will give a defibrillation shock to the patient or not after
analyzing the patient's ECG automatically.
(Defibrillation shock button should be pushed for delivering a
defibrillation shock.)
A non-rechargeable type lithium battery is used.
All-in-one type cartridge where electric pads and battery are
embedded is available. Also the detachable cartridge where electrode
pads and battery are separated is available.
Electrode pads can be used on both Adult and Pediatric patients.
(However, the attachment points are different.)
After analyzing ECG signals for 5 seconds, it stores them in the
internal memory (up to 1,000 cases during analysis only).
Stored data can be transmitted to a PC through the “NT-MPR” PC
management program.
Communication to PC is available with USB connection.
Ambient sound can be stored for up to four (4) cases and fifteen (15)
minutes for each case. (total memory: ~60 min.)
All operation procedures including CPR are guided by clear voice
prompts.
Self-diagnostics test function ensures device is in operational state.
Battery condition check in real time.
(In case of normal operational state, the pad attachment lamp
( ) flashes every 7 seconds.)

Section I -Product Introduction
12
Display Details
Product State
LED State
Description
Adult Mode
Adult mode lamp (Blue)
The adult LED light is lit
when the adult mode is on.
Pediatric Mode
Pediatric mode lamp
(Orange)
The pediatric LED light is lit
when the pediatric mode is
on.
Normal state
The normal state
(Green)
When battery power is
sufficient and device is
functioning normally, the
pads attachment lamp
flashes every 7 seconds.
Pads attachment
Attach the electrode
pads.
Before pads attachment:
Pads attachment lamp
flashes. After appropriate
pads attachment: the pads
attachment lamp stays on.

Section I- Product Introduction
13
ECG analysis
Analyze ECG.
During analysis: ECG
analysis lamp flashes
After completion of analysis:
ECG analysis lamp stays on.
Cardiopulmonary
resuscitation
Commence CPR.
(CPR lamp stays flashing)

Section I -Product Introduction
14
Name and Function of Components
No
Name/Shape
Function
①
Display
Power button (ON/OFF), two
separate buttons for either Adult
or Pediatric Modes, and lamps
indicating the current state of
device (or which step the user is
on).
②
Defibrillation shock
button (Red)
If the flashing defibrillation shock
button is pressed, a defibrillation
shock is delivered.
③
Power button for
Adult
The Adult Mode power button
prepares defibrillation shock mode
for adult.
Emission energy is 180J.
Adult
④
Power button for
Infant/Child
The Pediatric power button prepares
defibrillation shock mode for
infant/child.
Emission energy is 50J.
Note: Pediatric age range is from one
(1) to eight (8) years old or weight
is less than 25kg.
Pediatric
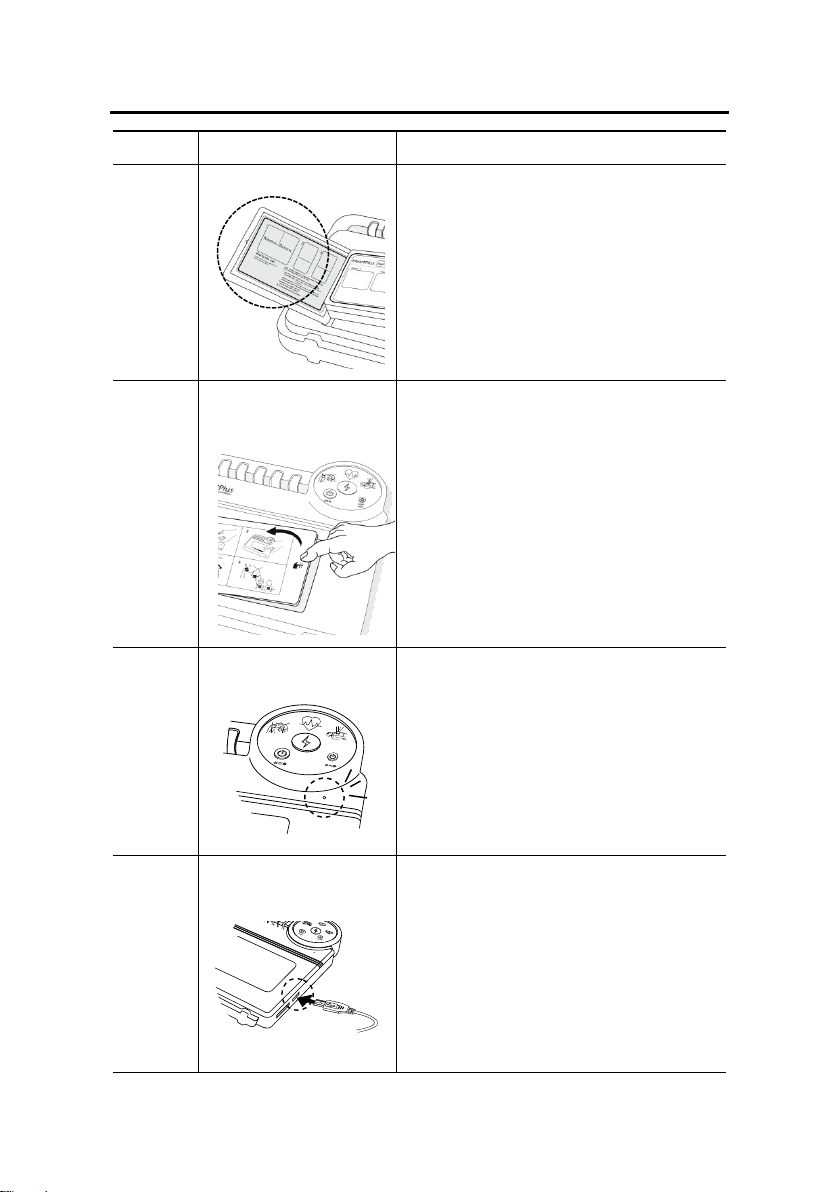
Section I- Product Introduction
15
No
Name/Shape
Function
⑤
Cartridge cover
A cover to open the case, revealing
the cartridge beneath.
⑤
PUSH then lift the
Cartridge Cover
To open the cover, push the hand
picture on the right side to release
and then lift the cover.
⑦
Microphone
Microphone to record ambient
sound during operation of product.
⑧
USB connection
Port for USB cable for data transfer
between HeartPlus™and PC
(Type B to Type A, respectively).

Section I -Product Introduction
16
No
Name/Shape
Function
⑨
Speaker
Plays voice prompts to guide user
through operation.
Alerts user of any problem
occurring during equipment
check or self-test via voice
prompt.
⑩
Handle
A handle to carry the equipment.
⑪
All-in-one type cartridge
Cartridge consists of two parts,
the electrode pads and the
battery.
When seal is opened, a pair of
electrode pads can be seen.
Battery incorporated in the
cartridge is below electrode pads
⑫
Electrode pads
Pads receive ECG signal and
delivers defibrillation shock when
attached to the patient, if needed.
Table of contents
Other AED Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Physiomed
Physiomed HIVAMAT 200 operating instructions

BD
BD Microtainer Quikheel quick start guide
Otto Bock
Otto Bock 14Y1 Patient Information

BioTel Heart
BioTel Heart MCOT Patch Patient guide

Harvest Healthcare
Harvest Healthcare OLYMPIC TURNER General User/ Safety Guide

Kimberly-Clark
Kimberly-Clark I-FLOW ON-Q PATIENT GUIDELINES