2
Table of Contents
CRISP Continuous Autofocus System....................................................................................... 4
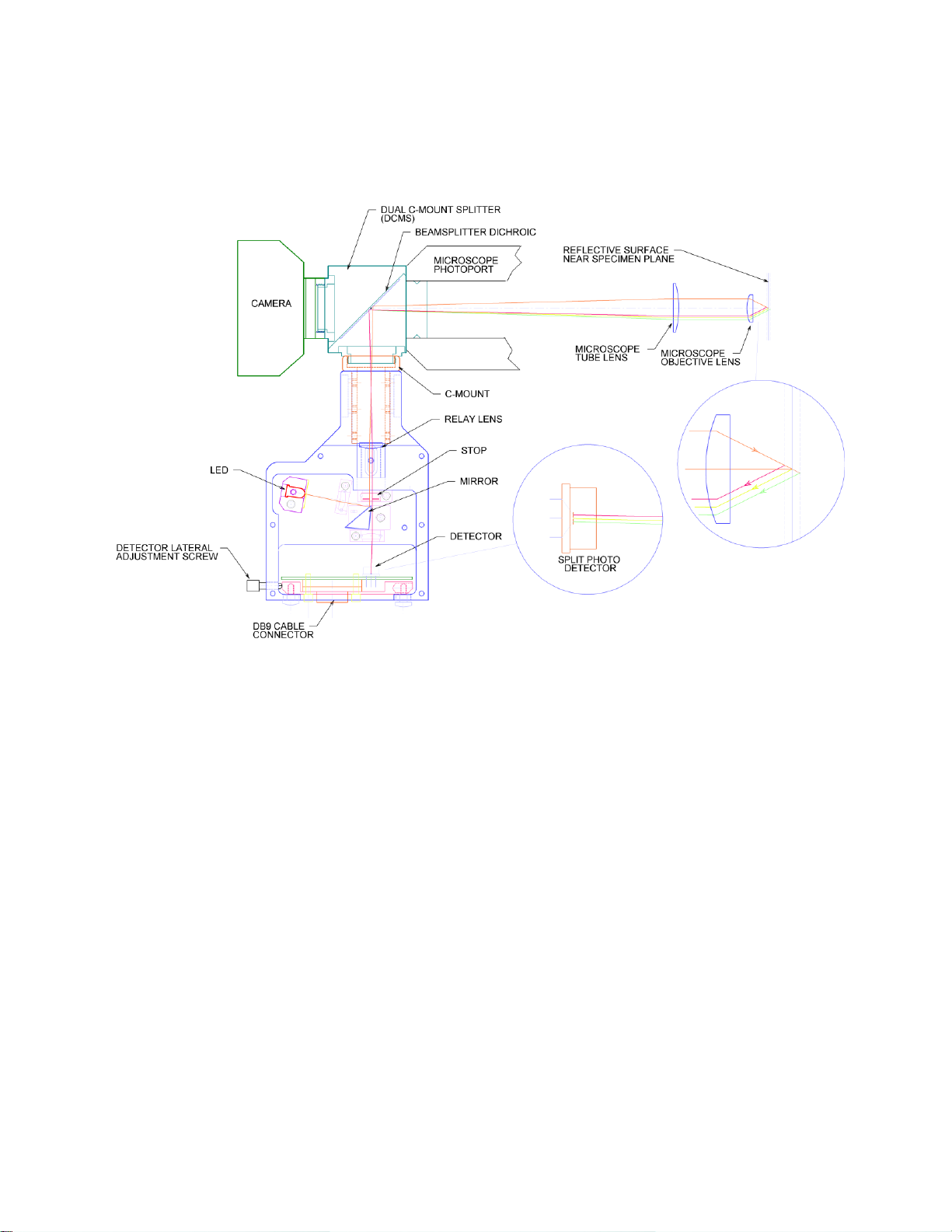
System Overview...................................................................................................................... 4
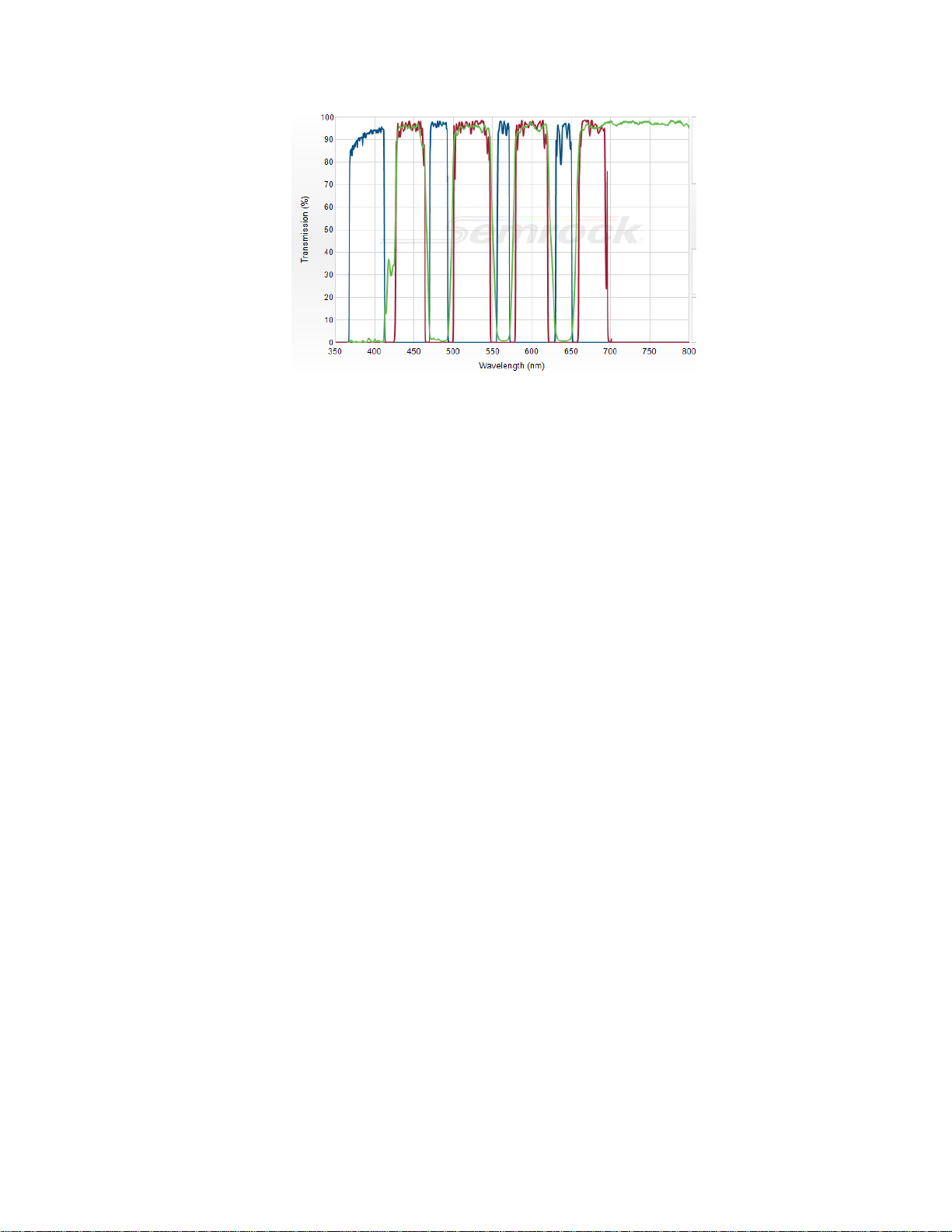
Fluorescent Filter Considerations.......................................................................................... 5
LED Characteristics and Filters ............................................................................................. 6
Commercial Filter Sets Suitable for CRISP........................................................................... 6
LED Power and Eye Safety..................................................................................................... 9
Installation.............................................................................................................................. 11
Theory of Operation.............................................................................................................. 11
Sample Considerations......................................................................................................... 11
Photodiode Displacement Signal.......................................................................................... 12
Control of the CRISP system ............................................................................................... 14
Button Actions...................................................................................................................... 14
CRISP System States............................................................................................................ 15
ASI Console support for CRISP ........................................................................................ 16
CRISP Operations................................................................................................................. 17
Quick Start Instructions Using ASI_Console....................................................................... 17
Quick Start Instructions Using Controller Only................................................................... 17
Engaging the LOCK for Normal Operation......................................................................... 18
Calibration Details................................................................................................................. 18
Optical Adjustment................................................................................................................ 20
Adjusting the Relay Lens position ....................................................................................... 20
Adjusting Position of the LED Light Source ....................................................................... 20
Adjusting the Primary Mirror............................................................................................... 21
Advanced Techniques............................................................................................................ 22
Troubleshooting Steps........................................................................................................... 25
Computer Control of the CRISP System .................................................................................. 26
TTL Control of the CRISP focus lock ................................................................................. 29