Atmos E 341 User manual

Operating instructions
ATMOS®E 341 Battery
English
2015-08 Index: 03
319.1000.B
319.1100.B

Table of contents
Further information, accessories, consumables and
spare parts are available from:
ATMOS
MedizinTechnik GmbH & Co. KG
Ludwig-Kegel-Straße 16
79853 Lenzkirch
Germany
Phone: + 49 7653 689-0
+ 49 7653 689-222 (Service Center)
Fax: + 49 7653 689-190
+ 49 7653 689-292 (Service Center)
www.atmosmed.de
2
1.0 Introduction. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
1.1 Notes on operating instructions ...................................... 4
1.2 Explanation of pictures and symbols .................................. 5
1.3 Intendeduseandsideeects ........................................ 6
1.4 Function .......................................................... 8
1.5 Intended users..................................................... 8
1.6 Scope of delivery ................................................... 9
1.7 Transport and storage ............................................. 10
2.0 Hints for your safety ............................................11
2.1 General safety information ......................................... 11
2.2 Danger for users, patients and third parties. .......................... 11
2.3 Damage to the device.............................................. 13
3.0 Setting up and starting up .......................................14
3.1 Device overview................................................... 14
3.1.1 Front and rear view............................................. 14
3.1.2 Control panel .................................................. 16

3
3.2 Preparing the device............................................... 17
3.3 Charging the battery............................................... 18
3.4 Connection and removal of canister system and hoses ................. 19
3.4.1 DDS canister system ............................................ 19
3.4.2 Serres®canister system ......................................... 22
3.5 Support for canister system ........................................ 23
3.5.1 DDS canister system ............................................ 23
3.5.2 Serres®canister system ......................................... 25
3.6 Hose rewind...................................................... 26
3.7 Device base ...................................................... 26
4.0 Operation......................................................28
4.1 Switch on the device ............................................... 28
4.2 Switchothedevice............................................... 28
4.3 Vacuum adjustment ............................................... 28
4.4 Suction .......................................................... 29
5.0 Cleaning and disinfection ........................................31
5.1 Prepare for cleaning ............................................... 31
5.2 Cleaning ......................................................... 31
5.3 After cleaning..................................................... 33
5.4 Recommended disinfectants........................................ 34
5.4.1 Instrument disinfectants ........................................ 34
5.4.2 Surface disinfectants............................................ 34
5.5 Cleaning and disinfection plan ...................................... 36
5.6 Oversuction ...................................................... 37
6.0 Maintenance and service ........................................38
6.1 Period tests ...................................................... 38
6.2 Function check.................................................... 38
6.2.1 Manual function check .......................................... 38
6.2.2 Automatic function check........................................ 39
6.3 Sending in the device .............................................. 40
6.4 Handling of batteries .............................................. 40
6.5 Battery exchange ................................................. 40
6.6 Release button exchange........................................... 42
7.0 Troubleshooting ................................................43
8.0 Accessories. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
8.1 Wall and device support............................................ 46
8.2 Retrotkitcanistersystem ......................................... 47
9.0 Spare parts and consumables ....................................48
10.0 Disposal .......................................................49
11.0 Technical data ..................................................50
12.0 Notes on EMC ..................................................53
4Introduction
1.0 Introduction
1.1 Notes on operating instructions
These operating instructions contain important notes on how to
operate the ATMOS®E341safely,correctlyandeectively.
These operating instructions serve not only for new operating
personnel to be instructed in its use, but also for use as a reference
manual. Any reprint - even in extracts - only after written permission
from ATMOS.
These operating instructions must always be kept available near
the device.
Care, period tests, regular cleaning and proper application are
indispensable. They guarantee the operational safety and usability of
the ATMOS®E 341 Battery.
Maintenance, repairs and period tests may only be carried out by
persons who have the appropriate technical knowledge and are
familiar with the product. To carry out these measures the person
must have the necessary test devices and original spare parts.
Prior to start-up please peruse chapter „2.0 Hints for your safety“
on page 11, in order to be prepared for any possible dangerous
situations.
The product ATMOS®E 341 bears CE marking CE 0124 according to
the EC Directive of the council for medical products 93/42/EEC and
meets the basic requirements of appendix I of this directive.
The product ATMOS®E 341 complies with all applicable requirements
of the directive 2011/65/EC restricting the use of certain hazardous
substances in electrical and electronic equipment (“RoHS”).
The declaration of conformity and our general standard terms and
conditions can be obtained on our website at www.atmosmed.com.
ThequalitymanagementsystemappliedatATMOShasbeencertied
according to international standards EN ISO 9001 and EN ISO 13485.
These operating instructions are valid for the following devices:
ATMOS®E 341 Battery / DDS REF 319.1000.0
ATMOS®E 341 Battery / Serres®REF 319.1100.0
SomeguresshowtheATMOS®C341Battery.However,thedevicesdonotdierin
their functionality described.
5
Introduction
1.2 Explanation of pictures and symbols
In these operating instructions
DANGER
Warning of a danger which can cause immediate death or serious injury. Observe the
necessary measures.
WARNING
Beware of a danger which can cause death or serious injury. Observe the necessary
measures.
CAUTION
Beware of a danger which can easily hurt you. Observe the necessary measures.
NOTICE
Indication of a danger where the product or other items can be damaged. Observe
the necessary measures.
Warning of a danger which can cause death or serious injury.
Information regarding possible material damage which can be caused.
Useful information on the handling of the device.
1. Action. Go step by step.
• Numeration.
»Result of an action.
Move, plug ... in this direction.
click
click
Engage,checkcorrectt.
On device and type plate
Follow operating instructions
(blue) Please observe operating
instructions
Manufacturer Manufacturing date
SN Serial number IP34D Type of protection
REF Order number KB Short-term operation
Professional disposal Application part type BF
Protection class II PATIENT Connection suction hose/patient
(Serres®canister system)
This product meets the
appropriate requirements of
the EU guidelines
2For single use only
(Symbol is on the consumables)
6Introduction
On the recharging accessories
Please observe operating
instructions
This product meets the appropriate
requirements of the EU guidelines
Manufacturing date Professional disposal
Manufacturer REF Order number
SN Serial number IP40 Type of protection
Output voltage
(13,8 V / 3,5 A)
Application part type CF
Input voltage
(100 - 240 V / 50 - 60 Hz /
1,1 A)
For indoor use only
Alternating current Direct current
Protection class II
On the battery
Please observe operating
instructions
This product meets the appropriate
requirements of the EU guidelines
Manufacturing date Professional disposal
Manufacturer REF Order number
SN Serial number EAN European Article Number
Mounting position: on the
top
Warning, special diligent notice
Donotthrowintore European Recycling Platform
1.3 Intended use and side e ects
Intended Use
Name: ATMOS®E 341 Battery
Main functions: Temporary and spontaneous suction of secretion, blood and body
uidsandalsoliquid,viscousandsolidpiecesoffoodinthemedicalsector.
Toevacuatevacuummattressesandinatablesplints.
Med. indications/ application: Suction of the upper and lower respiratory tract.
Speci cation of the main function: Drainage and temporary collection of body
uids.Bymeansofanelectricalsuctionpumpanegativepressurewillbecreated.The
integratedsuctioncanisterallowsatemporarilycollectionofthederivedbodyuids.
Application organ: Upper respiratory tract (nose, nasal cavity, throat), lower

7
Introduction
respiratory tract (larynx, trachea, bronchial system)
Application time: Temporary use on the patient (< 60 min.)
Application site: The application site is the clinic, the practice, the accident &
emergency department, the nursing and home care sector, as well as for outdoor
application and during transport. The application of the device may only be performed
bymedicallytrainedandintroducedsta.
Contraindications: Not suitable for
• The continuous operation by drainages in the low vacuum range (e.g. thorax
drainages or wound drainages).
• Permanent endoscopic use.
• Suction in medical rooms where a potential equalization is necessary (e.g. heart
surgery).
• Use outside the medical sector.
• Suctionofammable,corrosiveorexplosivesubstances.
• Suction in explosion-hazardous areas.
The product is: active
Sterility: The device is not sterile.
Single use product / reprocessing: The device and parts of the accessories are
reusable. For information on reprocessing and disinfection please see the operating
instructions.
Possible side eects during suction
• Bleeding in the nasal pharyngeal area
• Injury to the vocal cords
• Tracheal injury
• Hypoxemia
• Cardiovascular instability
• Bradycardia, Arrhythmia and Asystole (caused by vagus stimulation)
• Tachycardia (caused by stress)
• Choking, nausea, vomiting and coughing
• Nosocomial infection of the respiratory tract
• Seizures by patients who tend to develop cramps
Attentionmustbepaidtotheseoperatinginstructionsinordertokeepthesideeects
as minimal as possible.

8Introduction
1.4 Function
Der ATMOS®E 341 Battery is a mobile, portable, mains operated medical device for the
temporary application on adults, children and babies. The device is operated with an
electromotive, maintenance-free diaphragm pump.
The pump can be optionally operated by rechargeable battery or via an external DC
voltage source (12 V).
During operation the pump generates a vacuum within the hose system and collection
canister,thussuckingosecretion,bloodandbodyuidsaswellasliquid,viscousand
solidpiecesoffood.Theuidisgatheredinthecollectioncanister.
Thepredenedvacuumvaluesenablesaquickandpreciseadjustmentofthevacuum
indierentsituations.Itcanbeselectedbetweenfourdierentvacuumvalues(-0.1
bar; -0.2 bar; -0.5 bar und -0.8 bar). The control panel is illuminated, so that you can
read the operating status even after dark.
An overtemperature stop prevents overheating of the batteries.
DDS secretion canister:
TheDDSsecretioncanisterisaxedlaterallytothedeviceandispluggedviaDirect
Docking onto the suction connection at the support for the DDS canister system.
Therefore there is no intermediate hose. Now the user can/must only connect the
suctionhose.Ahydrophobicbacteriallterlocatedinthelidofthecanisterprevents
bacteria and liquids from entering the pump.
Amechanicaloversuctionstop(oatball)isintegratedinthecanisterlid.Thisprevents
anaccidentalabsorptionofsecretionintothepumphead.Theoatballrisestothetop
of the secretion until it blocks the outlet.
Disposable suction canister:
The disposable suction canister is comprised of an external canister, disposable suction
bag, vacuum hose and the disposable suction hose.
Thedisposablesuctioncanisterisaxedlaterallytothedevice.Thevacuumhose
of the canister is connected to the suction connection of the device. The secretion is
transported to the disposable suction bag via the suction hose. The disposable suction
bag is a single use product. As soon as the suction bag is full it is removed from the
external canister and disposed of. The disposable suction bag and the disposable
suction hose must not be reused.
Abacteriallterisintegratedinthedisposablesuctionbag.Thispreventssecretion,
liquid and bacteria from seeping into the device.
1.5 Intended users
The application of the ATMOS®E 341 Battery may only be performed by medically
trainedandintroducedsta.Priortoapplicationtheusermustbefamiliarwiththe
device.Attentionmustbepaidtocountry-specicrequirementsandregulations.
ATMOS recommends: instruction on the operation of the device must be performed by
an authorized person.

9
Introduction
1.6 Scope of delivery
1. Please compare the contents on completeness immediately upon receipt (see
delivery note).
Basic device
ATMOS®E 341
Battery with
device base
Hose rewind
(mounted)
Power supply
and recharging
unit 318.0035.0
2-pin mains
connection cable
008.0920.0
DDS canister system (mounted)
Support for
DDS canister
system
Reusable
suction hose,
Ø 10 mm,
L 1.3 m
Secretion
canister 1 l with
canister lid,
lterholder,
sealing ring
10 x Bacterial
lter
10 x Fingertip
Serres®canister system (mounted)
Support
for Serres®
canister system
Vacuum hose
with angled
connection
Serres®
external
canister 1 l
10 x disposable
suction hose with
ngertip,Ø6mm
Not included in the scope of delivery:
• Suction catheter
• Adapter for vacuum mattresses
• Serres®suction bag 1 l
• Wall and device support

10 Introduction
1.7 Transport and storage
Transportthedeviceonlyinashippingcarton,whichispaddedandoerssucient
protection.
If you notice any damage:
1. Document and report the transport damage.
2. Fill in the form QD 434 “customer complaint/return shipment”. This form is enclosed
to each delivery and can be found at www.atmosmed.com.
3. Send in the device to ATMOS (chapter „6.3 Sending in the device“ on page 40).
Ambient conditions during transport and storage:
• Temperature: - 40...+ 70 ° C
• Relative air humidity: 5...95 % without condensation
• Air pressure: 540...1100 hPa

11
Hints for your safety
2.0 Hints for your safety
The safety of the ATMOS®E 341 Battery complies with all the recognized rules of
technology and the guidelines of the Medical Products Law.
Please read and observe the safety instructions prior to using the product.
2.1 General safety information
Make yourself familiar with the device at an early stage, so you can use it even in hectic
situations.
Never operate the unit, if it shows any obvious safety defects. Check the unit at regular
intervals for safety and function.
2.2 Danger for users, patients and third parties.
Take care that the device is always functional and ready for use.
Yourpatientmaysuocate.
• Ensure that the device is always ready for use in an emergency.
• Position the unit in an easily accessible location and keep access free.
• Make sure that the charging accessories are functional. Replace defective charging
accessories immediately.
• Recharge the battery at the latest after 6 months, even if you do not use the device.
• Carry out a function check after each use. Carry out a function check every 4 weeks
in case you do not use the device for a longer period.
• ATMOS recommends always having another suction device ready to hand in case of
any device failure.
• Please observe the notes on electromagnetic compatibility (EMC) of the device.
Avoid misapplication.
Your patient may be seriously injured.
• The ATMOS®E 341 Battery may only be used by persons who were medically
trained, and were trained in the medical suction
• Please select the vacuum according to the patient and the application.
• Observe the valid guidelines.
Reduce the risk of infection for you and your patients!
Deadly diseases can be transmitted.
• Always wear disposable gloves, if you could come into contact with secretion.
• Never use components marked with 2more than once. These components are
intended for single use only.
• Only use sterile packaged parts, when the packaging is undamaged.
• Donotoperatethedevicewithoutabacteriallter.

12 Hints for your safety
Protect yourself against an electric shock!
Burns, cardiac arrhythmias and even death are possible.
• Do not operate the device if it has been dropped. In this case please clean the
device and send it in to ATMOS for repair.
• Disconnect the device from the mains power supply prior to cleaning or
disinfection.
• Prior to each use, please check whether the device or the recharging accessories
are damaged. Never operate the device if you detect any failure. In this case please
clean the device and send it in to ATMOS for repair.
• Take care that no liquid penetrates the device. In case that liquid has penetrated
the device it may no longer be operated. In this case please clean the device and
send it in to ATMOS for repair.
• The ATMOS®E 341 Battery cannot be sterilized.
• Use the recharging accessories in dry surroundings. The surroundings must be
non-conductive.
• Only use the recharging accessories according to the operating instructions.
• OnlyuseoriginalaccessoriesandoriginalsparepartsfromATMOS.Thisspecically
applies to the recharging accessories and the battery.
• Please pay attention to the period tests in chapter „6.0 Maintenance and service“ on
page 38.
• Assembly,repairs,modicationsandperiodtestsmayonlybecarriedoutby
authorized persons.
• Do not modify the device without permission of the manufacturer.
Explosion and re hazard!
Burns and injuries are possible.
• Neversuctionanyexplosive,ammableorcorrosivegasesorliquids.Pleasenote
theintendeduseinchapter„1.3Intendeduseandsideeects“onpage6.
• Never operate the device in explosion-hazardous areas or areas which are
oxygenated.
• OnlyuseoriginalaccessoriesandoriginalsparepartsfromATMOS.Thisspecically
applies to the recharging accessories and the battery.
Danger of suocation for children through accessories!
Childrencanstranglethemselvesorbesuocatedbysmallparts.
• Keep children away from hoses and connection cables.
• Keepchildrenawayfromswallowablesmallparts.Smallpartsare,e.g.ngertipand
sealing ring.
Tripping hazard by cables.
Injuries and fractures are possible.
• Lay connecting cables properly.
Only a fully functional product will meet the safety requirements of users, patients and
third parties. Please observe the following instructions carefully:

13
Hints for your safety
2.3 Damage to the device
Please observe the ambient conditions regarding transport, storage, operation and
recharging of the battery.
Take care that no liquid penetrates the device. In case that liquid has penetrated the
device it may no longer be operated. In this case please clean the device and send it in
to ATMOS for repair.
Alwaysplacethedeviceonrm,levelsurface.Thedevicemustalwaysbeinavertical
position, when you use it. Otherwise secretions may enter the unit.
Only use proper power connections and extension cords.
If possible, avoid a transport at temperatures below -5 °C. After transport in
temperatures below -5°C the device must be acclimatize for up to 6 hours at room
temperature before you continue with the next steps.
The device may only be connected to the mains power supply when mains voltage and
frequency of device and mains power supply correspond.
14 Setting up and starting up
3.0 Setting up and starting up
Pleaseobservethatinsucientbatterychargecanresultindamagetothebattery.
1. Thebatterymustbefullychargedpriortorstuse.
3.1 Device overview
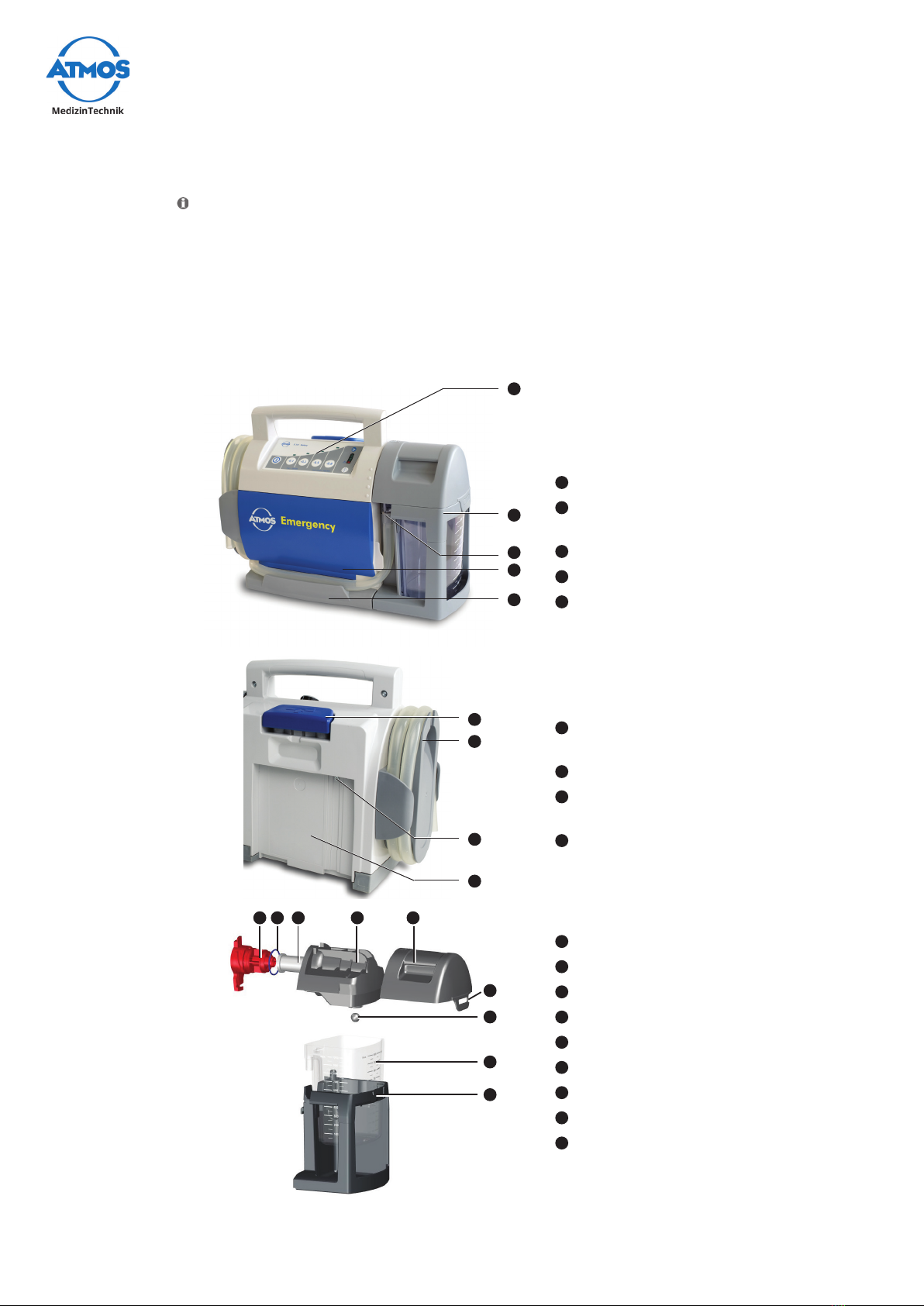
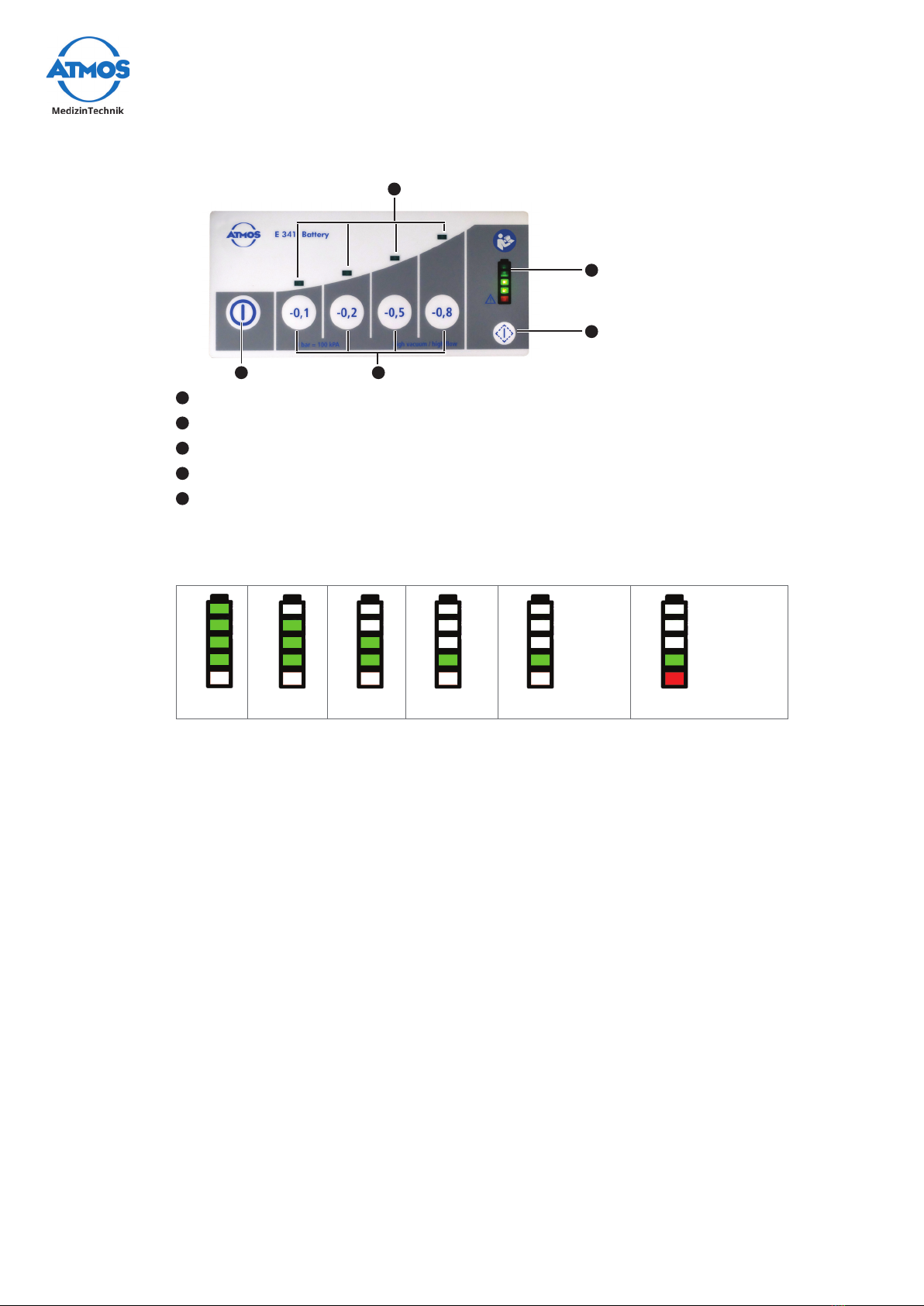
3.1.1 Front and rear view
With DDS canister system
1
2
3
4
5
8
9
6
7
1Control panel
2Support for DDS canister
system with DDS canister system
3Connection suction hose
4Battery compartment cover
5Device base
6Release button wall and device
support
7Hose rewind with suction hose
8Connection charging
accessories
9Guide for the wall and device
support
10 11 12 13 14
16
15
17
18
10 Filter holder
11 Sealing ring
12 Bacteriallter
13 Inner canister lid
14 Outer canister lid
15 Canister lid lug
16 Float ball
17 Secretion canister with scale
18 Support for DDS canister
system

15
Setting up and starting up
With Serres®canister system
1
2
3
4
5
8
9
6
7
1Control panel
2Connection disposable suction
hose
3Support for Serres®canister
system with Serres®canister
system
4Battery compartment cover
5Device base
6Release button wall and device
support
7Hose rewind with suction hose
8Connection charging
accessories
9Guide for the wall and device
support
9
10
11
12
13
14
9Angle (connection disposable
suction hose)
10 Serres®suction bag
11 Serres®external canister
12 Support for Serres®canister
system
13 Grey angle on the Serres®
external canister (connection
vacuum hose)
14 Vacuum hose with angled
connection
16 Setting up and starting up
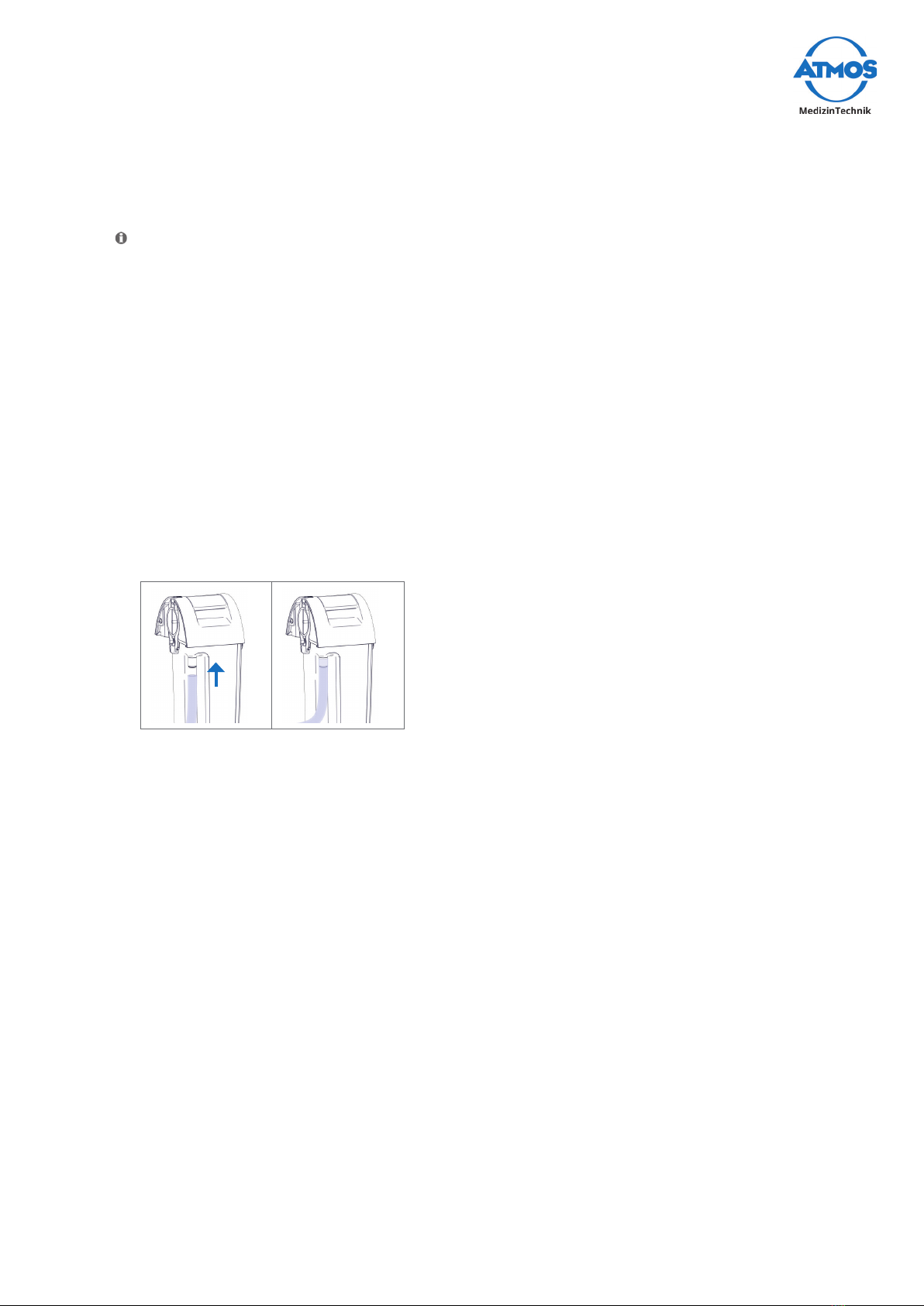
3.1.2 Control panel
1
2
3
45
1LEDs for the display of the current vacuum
2Battery status display
3Control button battery status
4Button to select the desired vacuum
5On/obutton
Display of the battery status
The following display values are not valid during battery charging.
1. 2. 3. 4. 5.
Flashes
6.
Green and
red LED
ash
>85% 60-85% 35-60% <15-35% <10-15% <10%
Prior to the battery going dead a signal tone sounds every 5 seconds.
AnerrorispresentifallthegreenLEDsashsimultaneouslyorallLEDsareashing.
Please observe chapter „7.0 Troubleshooting“ on page 43.
17
Setting up and starting up
3.2 Preparing the device
Priortorstoperationperusethesafetynotesinchapter„2.0Hintsforyoursafety“on
page 11.
Damaged pump diaphragms due to cold temperatures during transport.
1. After transport in temperatures below -5°C the device must be acclimatized for up
to 6 hours at room temperature before you continue with the next steps.
2. Check the device for any damage in transport.
3. If the device is damaged: Document and report the transport damage. Send in the
device to ATMOS (chapter „6.3 Sending in the device“ on page 40).
4. If the device is not damaged, place it on a safe and even surface.
5. Check the charging accessories for any damage.
6. Damaged charging accessories must be exchanged immediately.
7. The battery must be fully charged, chapter „3.3 Charging the battery“ on page 18.
8. Remove the canister system from the support.
9. WithDDScanistersystem:Priortorstuse,cleanthecanistersystemandinsert
abacteriallter(chapter„5.0Cleaninganddisinfection“onpage31aswellas
chapter „3.4 Connection and removal of canister system and hoses“ on page 19).
10. Connect the suction hose.
11. Place the canister system upright from above into the support: Chapter „3.4
Connection and removal of canister system and hoses“ on page 19.
12. Wrap the suction hose onto the hose rewind.
13. If you wish to use the device for vacuum mattresses: Check whether a suitable
adapter is available for the vacuum mattresses.

18 Setting up and starting up
3.3 Charging the battery
Thebatterystatuscanbecheckedbybrieypressingthecontrolbuttonforthebattery
status.
Thebatterymustbefullychargedpriortorstuse.
Damage to the battery due to deep discharge.
1. Charge the battery at the latest when the bottom green LED of the battery status
displayashes.
2. Only use the enclosed power supply and recharging unit 318.0035.0. Other charging
accessories must not be used.
3. Please observe the notes in chapter „6.4 Handling of batteries“ on page 40.
During battery recharging full suction performance of the device is still available.
If the battery is fully discharged or defective, the device may be operated via the
charging accessories.
If the ambient conditions are not adhered to, the charging time for the battery is
signicantlyincreased.Thechargingprocesswillbeterminatedifthetemperatureis
too high. Therefore, please prevent the device from direct solar radiation and keep it
away from radiators.
Ambient conditions during charging
• Temperature: +0...+40° C
• Relative air humidity: 5...95 % without condensation
• Air pressure: 540...1100 hPa
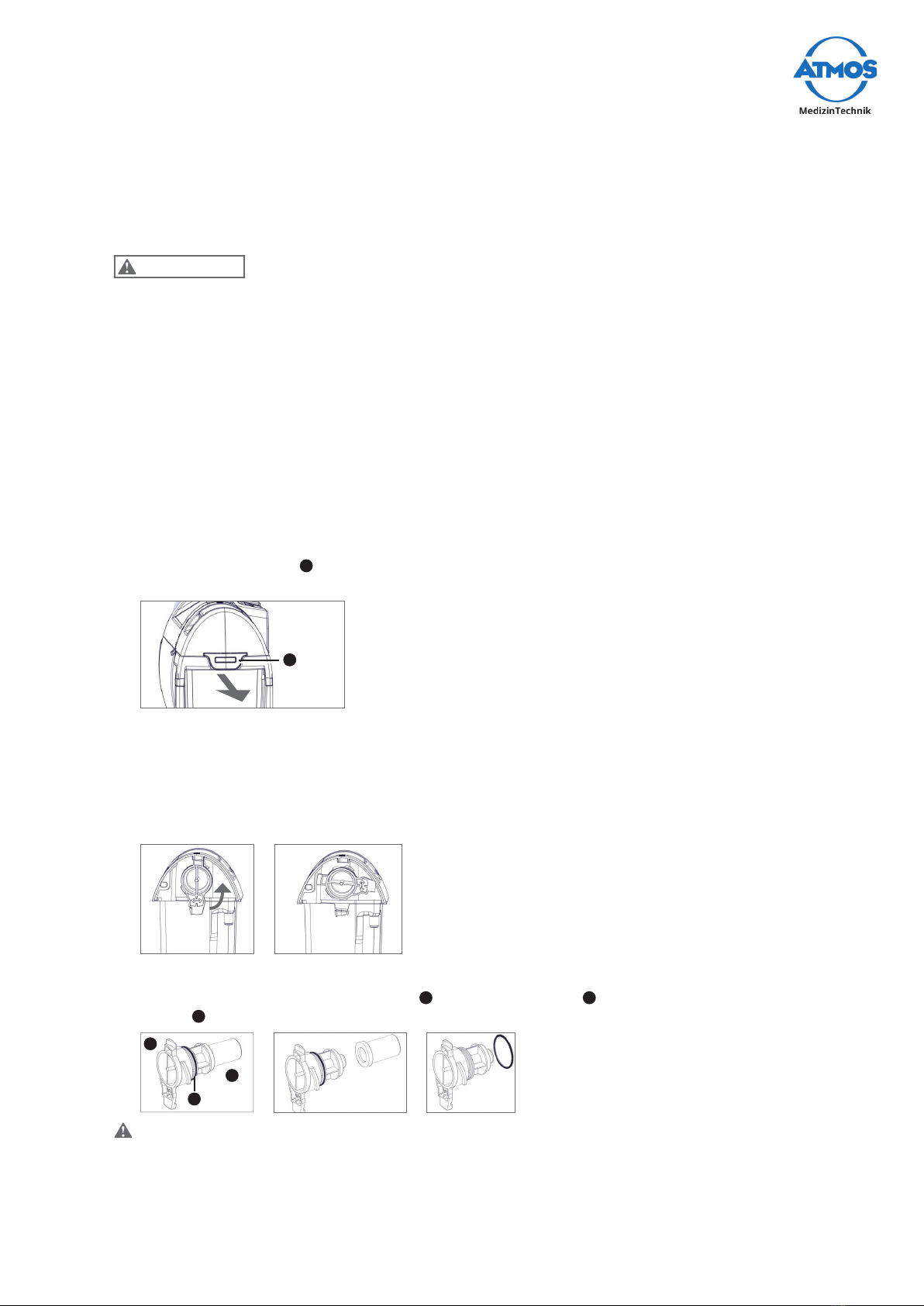
Charging with power supply and recharging unit
1. Connect the plug from the power supply and recharging unit to the back of the
device 1.
1
2. Connect the power cable to the power supply and recharging unit.
3. Plug in the power plug of the power supply and recharging unit to the socket.
»TheLEDsofthebatterystatusdisplayashsuccessively.
»One LED is continuously illuminated. This indicates the current battery status.
»The battery is fully recharged when the top red LED is continuously illuminated.
Recharging via the wall and device support
If you have attached the charging accessories to a wall and device support then the
device will be charged automatically: Chapter „8.1 Wall and device support“ on page
46.
1. Attach the device to the wall and device support.
»TheLEDsofthebatterystatusdisplayashsuccessively.
»One LED is continuously illuminated. This indicates the current battery status.
»The battery is fully recharged when the top red LED is continuously illuminated.
19
Setting up and starting up
3.4 Connection and removal of canister system and
hoses
3.4.1 DDS canister system
WARNING
Risk of infection by contaminated bacterial lter and canister lid.
Deadly diseases can be transmitted.
• Neverusethedevicewithoutabacteriallter.Werecommendyoualwaystostore
atleastonesparebacteriallter.
• Alwaysweardisposablegloveswhenchangingthebacteriallter.
• Priortoeachusepleasecheckwhetherthebacteriallterisdryandclean.Replace
awetorcontaminatedbacteriallterwithanewone.Abacterialltermayneverbe
reused.
• Exchangethebacteriallteraftereachuse.
Removal
1. Remove the suction hose from the hose rewind and then from the hose guide.
2. Gently unlock the clip 1of the canister lid from the support of the DDS canister
system and lift it upwards:
1
3. Lift the canister system upwards from the support.
4. Place the canister system on a safe and even surface.
5. Remove the suction hose from the secretion canister.
6. Turnthelterholderanti-clockwiseby90°.
Thelterholderisdiculttoturnbecauseithastosealthecanisterlidtightly.
7. Removethelterholderwithbacteriallterfromthecanisterlid.
8. Ifrequired:Removethebacteriallter 7and the sealing ring 6fromthelter
holder 5.
6
5
7
Riskofinfectionbyoverowingsecretion.Deadlydiseasescanbetransmitted.
9. Hold the secretion canister with one hand and pull it upwards with force.
»The canister system is open.
10. If required: Press the inner canister lid forward and remove it from the outer
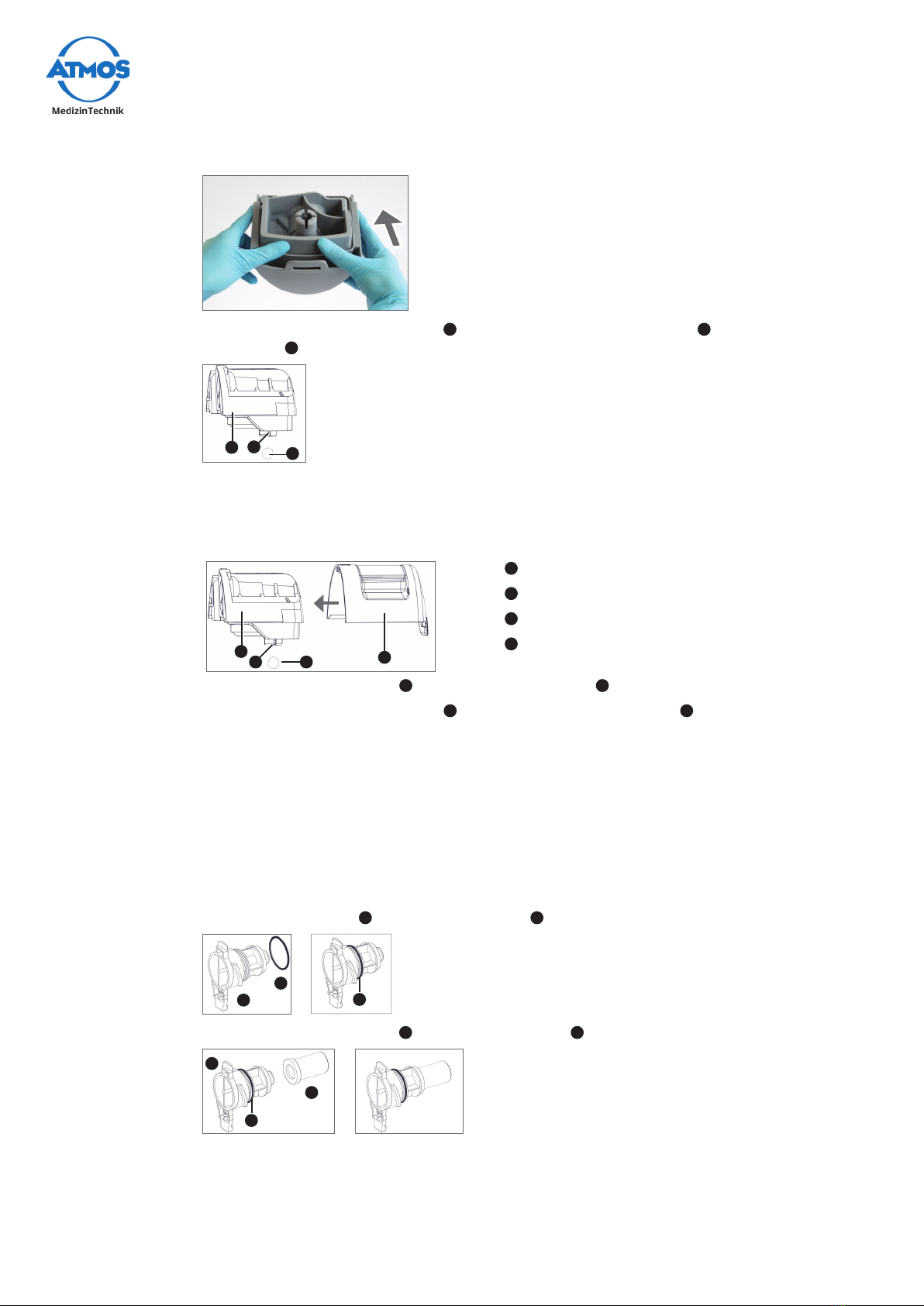
20 Setting up and starting up
canister lid.
11. Ifrequired:Removetheoatball 3fromtheoatballcompartment 2of the inner
canister lid 1.
123
Connection
When you pour 50-100 ml water or disinfectant into the secretion canister, then it is
easier to clean.
124
3
1Inner canister lid
2Float ball compartment
3Float ball
4Outer canister lid
1. Press the outer canister lid 4on the inner canister lid 1, until it clicks into place.
2. Opentheoatballcompartment 2gentlyandinserttheoatball 3.
3. Gentlypresstheoatballcompartmenttogether.
4. Checkwhethertheoatballmoveseasilyanddoesnotfalloutoftheoatball
compartment.
5. Placethesecretioncanisteronarmsurface.
6. Please observe the correct position of the canister lid prior to pressing it onto the
secretion canister.
7. Press the canister lid tightly with both hands as far as it will go onto the secretion
canister.
8. Place the sealing ring 6ontothelterholder 5.
6
56
9. Insertanewbacteriallter 7ontothelterholder 5.
5
7
6
Other manuals for E 341
1
Table of contents

















