BluePoint MEDICAL AlcoTrue E User manual

AlcoTrue® E - Operating instructions | bluepoint® MEDICAL GmbH & Co. KG 2014 1
Operating instructions
AlcoTrue
®
E
Breathalyser for personal use

AlcoTrue® E - Operating instructions | bluepoint® MEDICAL GmbH & Co. KG 2014 2
Liability
bluepoint MEDICAL GmbH & Co. KG shall under any circumstances not be liable for
any direct, indirect, special or consequential damages including without limitation
damages for loss of business profits, loss of income, business interruption, loss of
business information, loss of use or other related exposures, however caused, arising
from an incorrect use of the device not conforming to its intended use or/and if the
device is serviced and repaired by personnel not employed or authorized by bluepoint
MEDICAL GmbH & Co. KG.
bluepoint MEDICAL GmbH & Co. KG guarantees that the product delivered has been
thoroughly tested to ensure that it meets its published specifications.
AlcoTrue® is a registered trademark of bluepoint MEDICAL GmbH & Co. KG
Warranty
bluepoint MEDICAL GmbH & Co. KG warrants the products manufactured or distributed
by them to be free from faulty materials and workmanship for a period of 15 months
from date of original shipment to first end user except for wear parts, disposable
products or products which have a stated guarantee longer or shorter than 15 months.
bluepoint MEDICAL GmbH & Co. KG will perform warranty service at its factory.
The obligations for bluepoint MEDICAL GmbH & Co. KG under this guarantee shall be
limited to repair, or at bluepoint MEDICAL GmbH & Co. KG 's option, replacement of
necessary parts or assemblies and shall not include shipping costs or other incidental
damages.
Claims for damages during shipment must be filed promptly with the transportation
company. All correspondence concerning the products must specify both the name of
the product and its serial number as written on the label on the product.
Use of the equipment for other than its intended use, or if it has been repaired by
anyone except bluepoint MEDICAL GmbH & Co. KG or a bluepoint MEDICAL GmbH &
Co. KG authorized service centre, or altered or modified or used without following the
instructions in the user manual, will void this guarantee.

AlcoTrue® E - Operating instructions | bluepoint® MEDICAL GmbH & Co. KG 2014 3
Contents
1. Safety considerations ......................................................................................... 4
2. Intended use......................................................................................................... 5
3. Device................................................................................................................... 6
3.1 AlcoTrue® E - device components........................................................... 6
3.2 Device label-symbols................................................................................ 6
4. Battery information.............................................................................................. 7
4.1 Charging batteries..................................................................................... 7
4.2 Battery level indications............................................................................. 7
5. Device switch ON/OFF ........................................................................................ 7
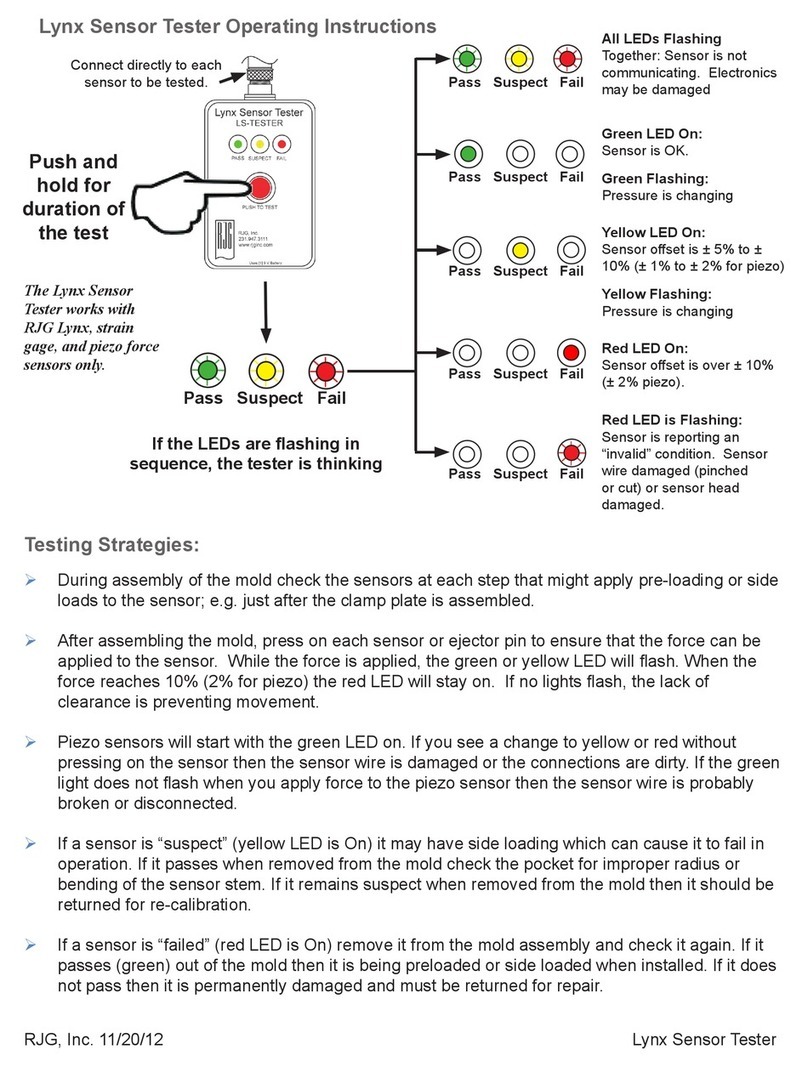
6. Carrying out the measurement........................................................................... 9
6.1Before measuring ...................................................................................... 9
6.1.1 Requirements of the person to be tested..................................... 9
6.1.2 Conditions................................................................................... 9
6.1.3 Inserting the mouthpiece........................................................... 10
6.2 Breath test procedure ............................................................................. 10
6.3 Error in delivering the breath sample ...................................................... 11
7. User menu and device settings........................................................................ 11
8. Maintenance, Cleaning, Disinfection ............................................................... 12
9. Disposal of the device....................................................................................... 12
10. Error /cause - solution..................................................................................... 13
11. Technical specifications ................................................................................. 14
12. Order list........................................................................................................... 16
AlcoTrue® E - Operating instructions | bluepoint® MEDICAL GmbH & Co. KG 2014 4
1. Safety considerations
Strictly follow the instructions for use
Handling the device requires full understanding and strict observation of these operating
instructions.
The device is intended only for the application described herein.
Maintenance
Calibration and maintenance work must be carried out by authorised service personnel
only. Only original parts from bluepoint MEDICAL GmbH & Co. KG may be used for
repairs.
Only the accessories specified in the order list shall be used.
Safety symbols
Important information on a topic or procedure, or conditions that may lead to
damage or malfunction of the device
Indicates potential harmful conditions that may lead to injury
Disposal
Do not dispose in the consumer waste. Electrical and electronic equipment
shall be collected and recycled in accordance with (Directive 2002/96/EC)

AlcoTrue® E - Operating instructions | bluepoint® MEDICAL GmbH & Co. KG 2014 5
2. Intended use
Intended use
AlcoTrue
®
E is a mobile breathalyser for personal use with 3x AA alkaline battery.
AlcoTrue
®
E is intended for quick and accurate determination of alcohol concentration in
a person’s breath.
NOTE: AlcoTrue®E is not an evidential measuring instrument and cannot be used
bindingly to determine if a person after use of alcohol can drive a vehicle or can operate
a machine. Measurements with AlcoTrue®E or similar kind of “ pre-test breathalysers”
are not a evidential proof of for example prior a court suitable. With AlcoTrue®E usage it
can only serve to confirm an initial suspicion.
Calibration
AlcoTrue®E is provided with a standard calibration by bluepoint MEDICAL GmbH & Co.
KG
The periods of calibration validity are 1000 measurements or 12 months, after
calibration period expires the device will require re-calibration. Using of AlcoTrue®E
with an expired device calibration has a risk of obtaining erroneous results.
The device can either be re-calibrated by bluepoint MEDICAL GmbH & Co. KG or an
authorised service partner.
The calibration overdue is displayed after device “Self test” procedure with <CAL>
Table of contents
Popular Test Equipment manuals by other brands

Redtech
Redtech TRAILERteck T05 user manual

Venmar
Venmar AVS Constructo 1.0 HRV user guide

Test Instrument Solutions
Test Instrument Solutions SafetyPAT operating manual

Hanna Instruments
Hanna Instruments HI 38078 instruction manual

Kistler
Kistler 5495C Series instruction manual

Waygate Technologies
Waygate Technologies DM5E Basic quick start guide

StoneL
StoneL DeviceNet CK464002A manual

Seica
Seica RAPID 220 Site preparation guide

Kingfisher
Kingfisher KI7400 Series Training manual
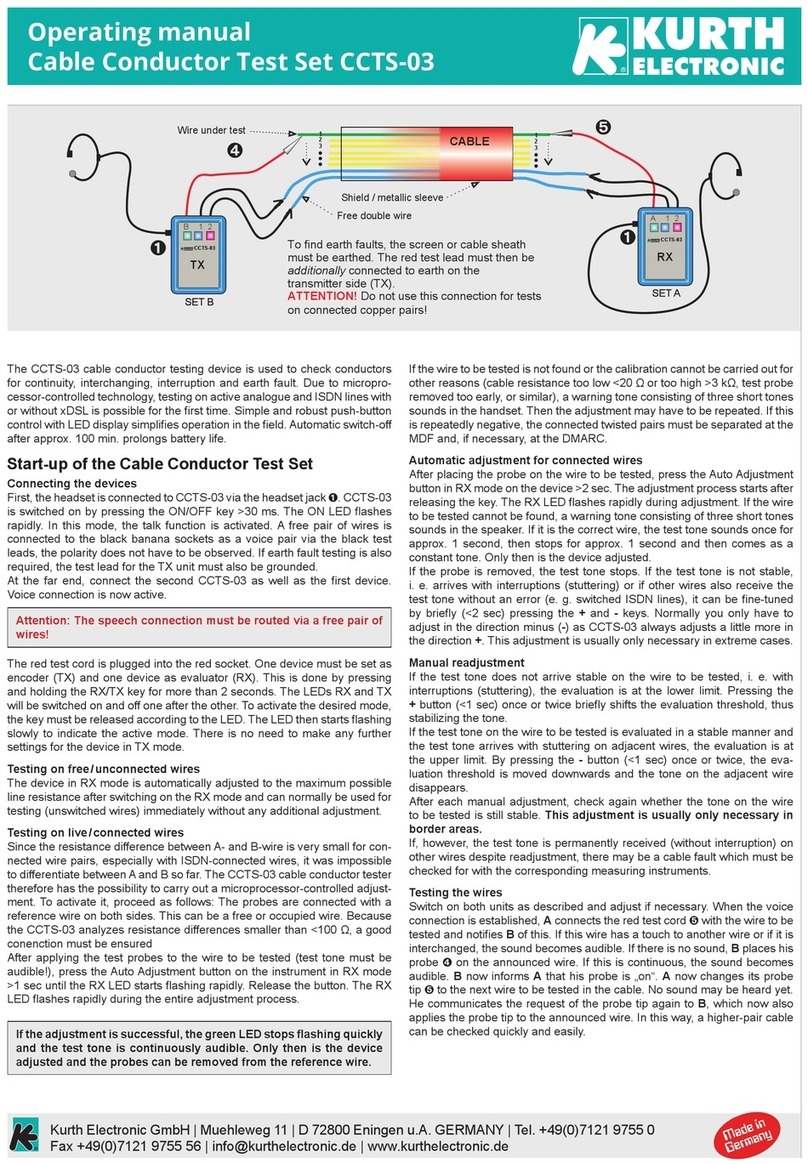
Kurth Electronic
Kurth Electronic CCTS-03 operating manual

SMART
SMART KANAAD SBT XTREME 3G Series user manual
Agilent Technologies
Agilent Technologies BERT Serial Getting started