3
Contents
7 Operation ...................................................................................................... 23
7.1 Starting a new therapy ..........................................................................................23
7.2 Calculation of new suggested values ................................................................26
7.2.1 Hypoglycaemia warning ...........................................................................27
7.2.2 Blood glucose value is not plausible .....................................................27
7.2.3 Error in calculation .....................................................................................27
7.3 Conrming the suggested insulin dose rate / SGC mode.............................28
7.4 Entering the blood glucose value ........................................................................28
7.5 Overruling the insulin dose rate / manual mode ............................................29
7.6 Returning to the SGC mode ..................................................................................30
7.7 Changing the nutrition rate (enteral / parenteral) ........................................30
7.8 Stopping nutrition (enteral / parenteral) / interrupting
for short period ........................................................................................................30
7.9 Entering parenteral bolus ...................................................................................... 31
7.10 Entering a meal ........................................................................................................31
7.11 Enabling standby mode on the device as well as insulin and
nutrition pumps .......................................................................................................32
7.12 Data Lock ...................................................................................................................32
7.13 Displaying the insulin pump settings .................................................................32
7.14 Displaying the nutrition pump settings (enteral / parenteral) ...................33
7.15 Stopping the insulin pump ....................................................................................33
7.16 Ceasing the therapy ................................................................................................34
7.17 Continuing the therapy ..........................................................................................34
7.18 Starting a new therapy after a therapy has been ceased ............................34
7.19 Steps to be taken if the SGC therapy does not start .....................................35
8 Alarms .......................................................................................................... 37
9 Cleaning and care / Disposal ........................................................................ 38
9.1 Cleaning and care ....................................................................................................38
9.2 Disposal ......................................................................................................................38
10 Technical Safety Check (TSC) / Service ....................................................... 39
11 Guarantee ...................................................................................................... 39
12 Technical data ................................................................................................ 40
13 Instructions on electromagnetic compatibility (EMC)............................... 41
14 Order data ...................................................................................................... 43
Contents
1 About this document........................................................................................4
1.1 Purpose ......................................................................................................................... 4
1.2 Additional valid instructions for use .................................................................... 4
1.3 Markings and symbols .............................................................................................. 4
1.4 Abbreviations .............................................................................................................. 4
2 Safety instructions ...........................................................................................5
2.1 Intended use ............................................................................................................... 5
2.2 Safe handling of the device .................................................................................... 5
2.3 Safe application of the automated glucose monitoring ................................ 7
2.4 Other components ..................................................................................................... 8
2.5 Safety standards ........................................................................................................ 8
3 Description of the device .................................................................................9
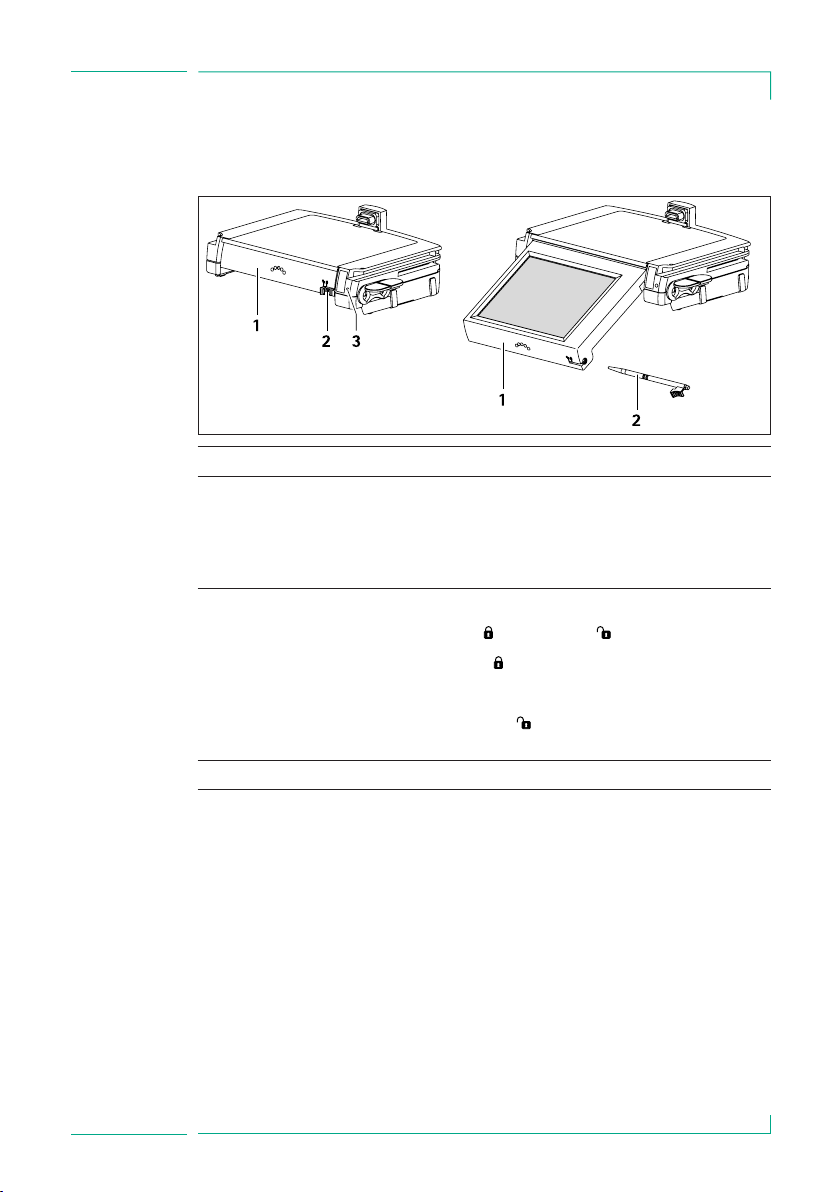
3.1 Displays and controls ................................................................................................ 9
3.2 Interfaces ...................................................................................................................10
3.3 Symbols on the device ...........................................................................................10
3.4 Application of the automated glucose monitoring .........................................11
3.4.1 Positioning of the device within the SGC system /
compatible components .............................................................................11
3.4.2 Tasks of the device within the SGC system ..........................................12
4 Description of the functions / Operation .................................................... 13
4.1 Screen design ............................................................................................................13
4.1.1 Overview screen (example) .......................................................................14
4.1.2 Display of alarm states ...............................................................................15
4.2 Navigation and input elements ...........................................................................16
5 Menu structure / Overview of functions .................................................... 18
5.1 Main menu ................................................................................................................18
5.2 Nutrition .....................................................................................................................18
5.3 Options........................................................................................................................19
5.3.1 Options > Patient data ...............................................................................19
5.3.2 Options > System Data > System Data .................................................19
5.3.3 Options > System Data > SpaceCom .....................................................20
5.3.4 Options > Conguration SGC ...................................................................20
5.3.5 Options > Conguration SpaceControl .................................................21
5.4 Start / Stop and Overview .....................................................................................21
6 Start-up and self-test................................................................................... 22