dental X dxp DOMINA PLUS B User manual

OPERATOR MANUAL
USE and MAINTENANCE
Steam sterilizer
Domina Plus B_UM_EN
Rev. 12
Date: October 2019
OM1004U

2

3
TABLE OF CONTENTS
1 GENERAL INFORMATION....................................................................................5
1.1 Purpose of the manual................................................................................................................................................................................... 5
1.2 Criteria for use of the manual and finding information ..................................................................................................................... 5
1.3 Professional user profiles............................................................................................................................................................................... 6
1.4 Conformity to European Directives ........................................................................................................................................................... 6
1.5 Warranty .............................................................................................................................................................................................................. 6
2 SAFETY INFORMATION .......................................................................................7
2.1 General safety information........................................................................................................................................................................... 7
2.2 Safety and protection features on the device........................................................................................................................................ 8
2.2.1 Soft-close door with double safety.............................................................................................................................................................8
2.2.2 Overpressure protection - safety valve and pressure relief valve ........................................................................................................8
2.2.3 Blackout protection........................................................................................................................................................................................8
2.2.4 Overheating protection ................................................................................................................................................................................8
2.2.5 Automatic power off......................................................................................................................................................................................8
2.3 Safety signs on the device ............................................................................................................................................................................ 9
2.4 Residual risks....................................................................................................................................................................................................10
2.5 Bacteriological risks.......................................................................................................................................................................................10
3 CHARACTERISTICS ............................................................................................11
3.1 Description of the sterilizer ........................................................................................................................................................................11
3.2 Intended use....................................................................................................................................................................................................11
3.3 Environmental conditions...........................................................................................................................................................................11
3.4 Units that make up the sterilizer...............................................................................................................................................................12
3.5 Components supplied with the sterilizer..............................................................................................................................................14
3.6 Size and weight of package........................................................................................................................................................................15
3.7 Size and weight of sterilizer........................................................................................................................................................................15
3.8 Technical specifications ...............................................................................................................................................................................16
3.9 Sterilizer regulatory label ............................................................................................................................................................................17
4 INSTALLATION ...................................................................................................20
4.1 Unpacking and transportation..................................................................................................................................................................20
4.2 Positioning........................................................................................................................................................................................................21
4.3 Initial start-up ..................................................................................................................................................................................................23
4.4 Compensating for altitude..........................................................................................................................................................................24
4.5 Setting date and time...................................................................................................................................................................................25
4.6 Setting temperature and pressure measurement units and selecting the language...........................................................25
5USING THE STERILIZER ................................................................................... 26
5.1 Description of the operator panel ...........................................................................................................................................................26
5.2 Turning the sterilizer on...............................................................................................................................................................................27
5.3 Daily tests to check the sterilizer's performance ................................................................................................................................27
5.3.1 Vacuum test ................................................................................................................................................................................................. 27
5.3.2 Helix test and Bowie & Dick test ............................................................................................................................................................... 27
5.4 Preparing the material before sterilization...........................................................................................................................................28
5.4.1 Preliminary operations............................................................................................................................................................................... 28
5.4.2 Treatment of materials and instruments before sterilization........................................................................................................... 28
5.5 Arranging the material on the trays before sterilization..................................................................................................................29
5.6 Program selection..........................................................................................................................................................................................30
5.7 Running the program...................................................................................................................................................................................31
5.8 Interrupting the programme.....................................................................................................................................................................32
5.9 Setting the CUSTOM - S5 program ..........................................................................................................................................................33
5.10 Topping up with demineralized water and draining contaminated water...............................................................................34
5.10.1 Topping up the demineralized water tank ............................................................................................................................................ 34
5.10.2 Draining the contaminated water recovery tank................................................................................................................................ 34
5.11 Diagnostics.......................................................................................................................................................................................................35
5.11.1 Manual diagnostics..................................................................................................................................................................................... 35
5.11.2 Power-on automatic diagnostics ............................................................................................................................................................ 35
5.11.3 Checking water quality .............................................................................................................................................................................. 35

4
TABLE OF CONTENTS
5.12 Connections.....................................................................................................................................................................................................36
5.12.1 Connection to an external printer ........................................................................................................................................................... 36
5.12.2 Integrated printer (optional)..................................................................................................................................................................... 37
5.12.3 Connection to a USB log (optional)......................................................................................................................................................... 37
6 ALARMS ..............................................................................................................38
6.1 Overview ...........................................................................................................................................................................................................38
6.2 List of warning messages............................................................................................................................................................................38
6.3 List of alarms....................................................................................................................................................................................................39
7 MAINTENANCE...................................................................................................40
7.1 Periodic maintenance...................................................................................................................................................................................40
7.2 Sterilization chamber automatic cleaning cycle.................................................................................................................................40
7.3 Cleaning or replacement of the demineralized water filter............................................................................................................41
7.4 Replacing the bacteriological filter..........................................................................................................................................................42
7.5 Cleaning the instruments before sterilization .....................................................................................................................................42
7.6 Schedulled maintenance.............................................................................................................................................................................42
5
GENERAL INFORMATION - 1
1 GENERAL INFORMATION
1.1 Purpose of the manual
This operator manual was issued by NSK Dental Italy to provide the operator with the necessary information for:
proper installation
appropriate and safe use
careful maintenance
The manual is an integral part of the Domina Plus B steam sterilizer, hereafter referred to in this manual as the “sterilizer" or,
more simply, the "device", and must always remain with it, even when sold.
It should always be kept close to the device, in an easily accessible place and protected from environmental agents that
could affect its integrity and durability. It should readily at hand for immediate consultation at any time by operators and
maintainers.
Read the manual carefully and understand it fully before installing the device and putting it into service, particularly the
instructions given in the chapter on “Safety information”, which are aimed at preventing potential risks that could cause
injuries to the operator or damage to the device.
The company that uses the devices is responsible for always ensuring that all operators fully understand the operating
instructions.
DENTAL X declines any responsibility for failure to observe the safety and prevention rules described in the various sections
of this manual and for damages caused by improper installation and use of the device.
All rights are reserved.
This publication may not be reproduced, transmitted, transcribed, stored in computer systems or translated into another
language or computer language, even partially, in any form or by any means without prior written permission from NSK
Dental Italy.
NSK Dental Italy reserves the right to make changes to the technical characteristics of the product described in this manual
at any time, with no obligation of prior notice or communication.
1.2 Criteria for use of the manual and finding information
The information and instructions are collected and organized into chapters and paragraphs, and can be easily found by
searching the index.
Information preceded by a warning sign must be read carefully.
Basic information for the health and safety of operators/maintenance personnel is contained in a box marked with warning
signs on a coloured background, as illustrated below.
Safety instructions are classified as follows, in accordance with the seriousness of the risk:
Classification Risk level
NOTE Information on general product specifications highlighted to prevent malfunctions and loss
of product performance.
CAUTION
Indicates cases where failure to follow the safety instructions may lead to minor or
considerable injury to people or damage to the device.
WARNING
Indicates cases where failure to follow the safety instructions may lead to serious injury to
people or damage to the device.

6
GENERAL INFORMATION - 1
1.3 Professional user profiles
European regulations on safety and the sterilization process describe the following professional roles:
OPERATOR a person who uses the device for the intended purpose on a daily basis
MAINTENANCE TECHNICIAN a person assigned to the ordinary maintenance of the device on a daily basis
Note: the operator and maintenance technician may also be the same person.
RESPONSIBLE AUTHORITY: an individual (often the employer) or group of people responsible for the use and maintenance
of the device, who ensures that:
• the operator and maintenance technician are adequately trained to use the device in full safety;
• regular training is provided for all personnel regarding the operation and maintenance of the device, including emergency
procedures in the event of emission of toxic, flammable, explosive or pathogenic material into the environment;
• registration documents for attendance of the training are preserved and its full understanding is verified;
• a written, electronic or paper record is kept of the sterilization procedures carried out from the moment the device is
installed.
1.4 Conformity to European Directives
The Domina Plus B sterilizer produced by NSK Dental Italy satisfies the essential requirements of the Directive 93/42/EEC for
medical devices, European Standard EN 13060 and Directive 2014/68/EC for pressure equipment (PED).
This DENTAL X product has been designed and manufactured with high quality materials and parts that can be recycled and
reused.
Separate disposal of electrical and electronic equipment, in accordance with Directive 2012/19/UE (WEEE).
The equipment belongs to Category 8 (medical equipment). In force in the nations of the European Union,
Norway and Switzerland.
CE Mark and Notified Body number.The CE mark indicates that the device satisfies the essential requirements
of the Medical Devices Directive 93/42/EEC.
Notified Body: IMQ S.p.A., Via Quintiliano, 443, 20138 Milan (Italy), Identification N. 0051.
1.5 Warranty
DENTAL X products are guaranteed against manufacturing errors and defective materials. NSK Dental Italy reserves the right
to examine and determine the cause of any problem. The warranty will be void if the product has not been used properly or
for its intended use, if it has been tampered with by unqualified personnel or fitted with non-original NSK Dental Italy parts.
Replacement parts are available for ten years after production of the model has ceased.
Failure to follow the guidelines given below will void the warranty and/or make the device dangerous to operate.
In the event of faults and/or malfunction, follow the guidelines given in paragraph 6.2 “Warning messages” and paragraph
6.3 “List of alarms”. If the problem persists, do not attempt to operate the device but contact the NSK Dental Italy technical
support.
Do not operate the device until the necessary repairs have been made to restore its proper operation.
Do not attempt to disassemble the device, replace faulty or damaged components and/or have it adjusted or repaired by
personnel without proper training and authorization from NSK Dental Italy.
Faulty or damaged components should only be replaced with original NSK Dental Italy parts.
7
SAFETY INFORMATION - 2
2 SAFETY INFORMATION
2.1 General safety information
To maintain a maximum level of device safety for patients and specialized professional operators, it is essential that:
the operators and maintenance technicians have read and understood the instructions for installation and use of the
device
the periodic maintenance operations described in the chapter 7 “Maintenance” are carried out
the following safety instructions are observed:
WARNING
• Ensure that the device is connected to a power socket with an earth connection.
• Keep the plug in the socket until the sterilization is finished and do not use the socket for other devices at the same
time.
• Use only original NSK Dental Italy power cables, as other cables can cause electric shock, fires or damage to the device.
• Do not turn the power on or off unless strictly necessary, as this may trip the fuse.
• Do not touch the power cord with wet hands as this may cause electric shock.
• Install the product with sufficient space to allow immediate removal of the electrical plug.
• Turn off the power switch and disconnect the power cord before performing any maintenance.
• Do not connect non-original NSK Dental Italy accessories or equipment to the device.
• Keep explosive substances and flammable materials far from the device.
• If the device overheats or emits a bad smell, turn off the power switch immediately, remove the plug from the electrical
socket and contact technical support.
• Do not allow water or disinfectant liquid to enter the inside of the device as it may cause a short circuit and electrical
shock.
• Avoid inadvertently touching the door or the area around the chamber while the device is in operation or immediately
after stopping the product, as these reach high temperatures and can cause burns.
• Do not obstruct or cover the steam outlet on the product with other objects. In addition, avoid inadvertently placing
your face or hands near the steam outlet, as this can cause burns.
• Only use NSK Dental Italy original components and spare parts.
CAUTION
• The device must only be installed in enclosed environments.
• Install the machine on a flat surface.
• Do not sterilize liquids or objects other than medical instruments reported in the intended use.
• Avoid any impact on the device. Do not drop the device.
• Wash and dry objects before sterilization. Chemical detergent residues in the chamber can cause corrosion or leave
bad odours on sterilized objects.
• Insert the objects to be sterilized using the racks. Directly inserting objects into the chamber may cause sterilization
problems, discolouration or even damage to the objects.
• Ensure that any water has been drained before moving the device.
• Use a container or case for sterilizing fine-pointed objects, as these may protrude from the bottom of the rack.
• Sterilize the instruments in accordance with the parameters recommended by the manufacturer or retailer.
• If any irregularities are noticed during use, stop the sterilization cycle and contact technical support.
• Conduct periodic diagnostic checks and routine maintenance operations.
• If the device has not been used for a long time, check that it is working properly before use.
• Portable and mobile RF communication devices can interfere with the device.
• The device must not be used near or above another device. If this is not possible, ensure that all devices work properly.
• The device may malfunction if used near electromagnetic interference. Do not install the device near other equipment
that emits magnetic waves. Turn off the power if an ultrasonic oscillation or electrosurgery device is located near the
site of use.

8
SAFETY INFORMATION - 2
2.2 Safety and protection features on the device
The sterilizer has several devices, listed below, that ensure the total safety of operators.
2.2.1 Soft-close door with double safety
An electromechanical device allows the door to be opened only under the following conditions:
• device plugged in and switched on
• no alarms activated
• internal pressure not hazardous to the operator
For additional safety, the Start/Stop button must be pressed to unlock the door at the end of a cycle.
CAUTION
If the device is switched off with the door open, do not try to close the door by forcing the
handle. To close the door, simply turn the device on again using the main switch.
2.2.2 Overpressure protection - safety valve and pressure relief valve
Safety valve
This is a valve located on the back of the device that is triggered when the pressure inside the
chamber exceeds 2.55 bar. To check that valve is working properly, switch the device off and allow
it to cool down, then unscrew the black cap, pull it slightly until a "click" is heard and then check
that it moves freely. The safety valve requires no adjustment or maintenance.
Pressure relief valve
This is triggered when the pressure inside the sterilization chamber exceeds 2.4 bar; an acoustic
signal alerts the operator and the message ALARM 16 appears on the display.
2.2.3 Blackout protection
In the event of a power supply failure during the sterilization cycle, the pressure in the chamber
is completely released and brought down to ambience level. When the power supply returns, the
message BLACK OUT appears on the display.
2.2.4 Overheating protection
The temperature inside the sterilization chamber is programmed to not exceed a limit of 142 °C; in the event of failure,
additional protection is provided to prevent the temperature from rising above 150 °C.
2.2.5 Automatic power off
Thirty minutes after the end of the cycle, unless the door has been opened or a button pressed on the front panel, the device
automatically switches off.
NOTE This function is not implemented if no sterilization cycle has been run.
9
SAFETY INFORMATION - 2
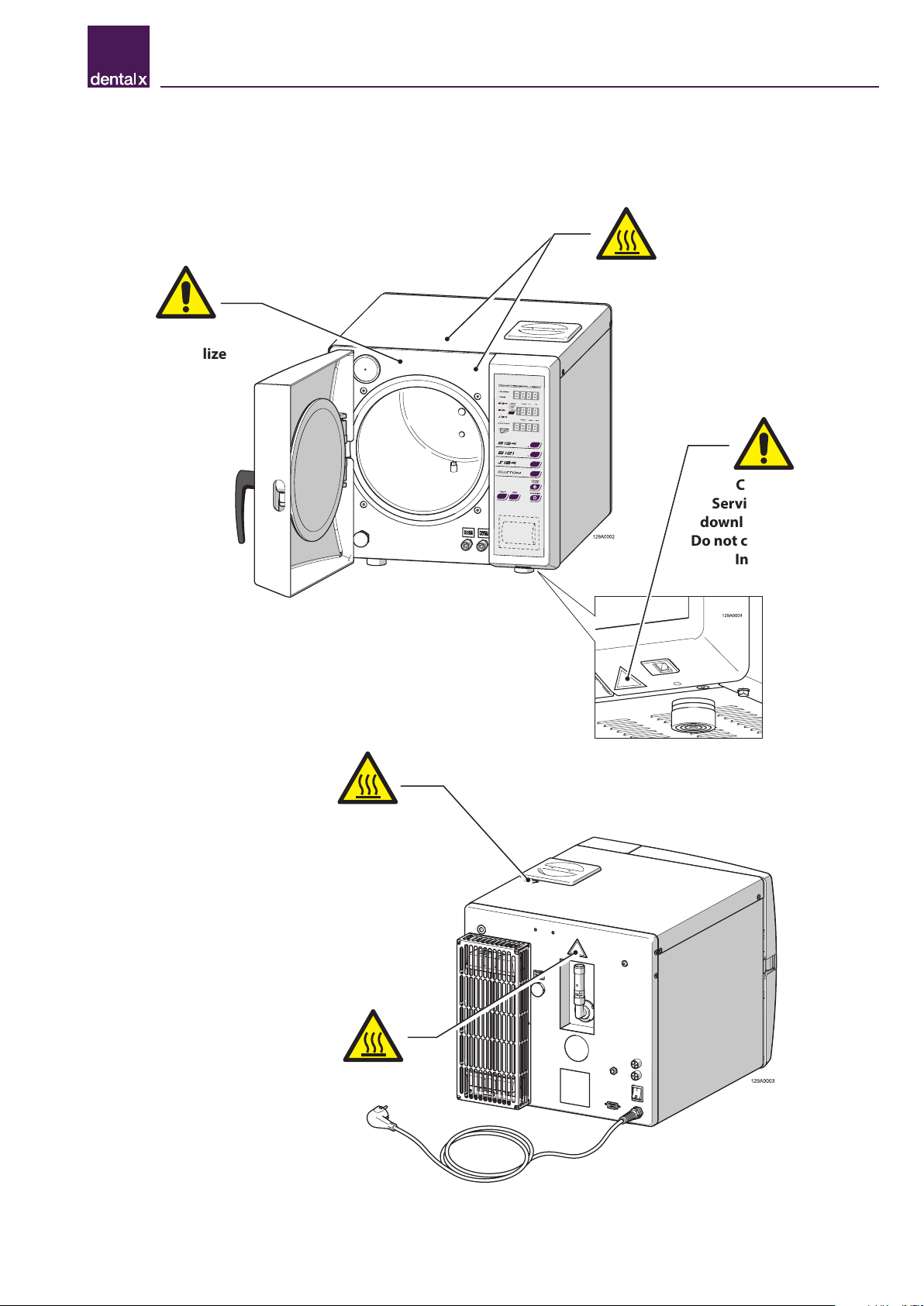
2.3 Safety signs on the device
The following warning and hazard signs are located on the sterilizer in the positions indicated.
Caution!
Hot surfaces.
Use only demineralized
water.
Tank capacity 4 litres.
Caution! Hot surfaces.
Caution!
Do not sterilize
fluids.
Caution!
Service port for
downloading data.
Do not connect to the
Internet.
Caution! Hot surfaces.
10
SAFETY INFORMATION - 2
2.4 Residual risks
The sterilization process works by means of pressurized steam at high temperature. When removing a load from the
sterilization chamber, always use suitable tools and personal protective equipment for handling the hot racks and tools.
When opening the sterilizer door, particularly during a cycle failure, a small quantity of steam or hot condensate may be
released; open the door with caution.
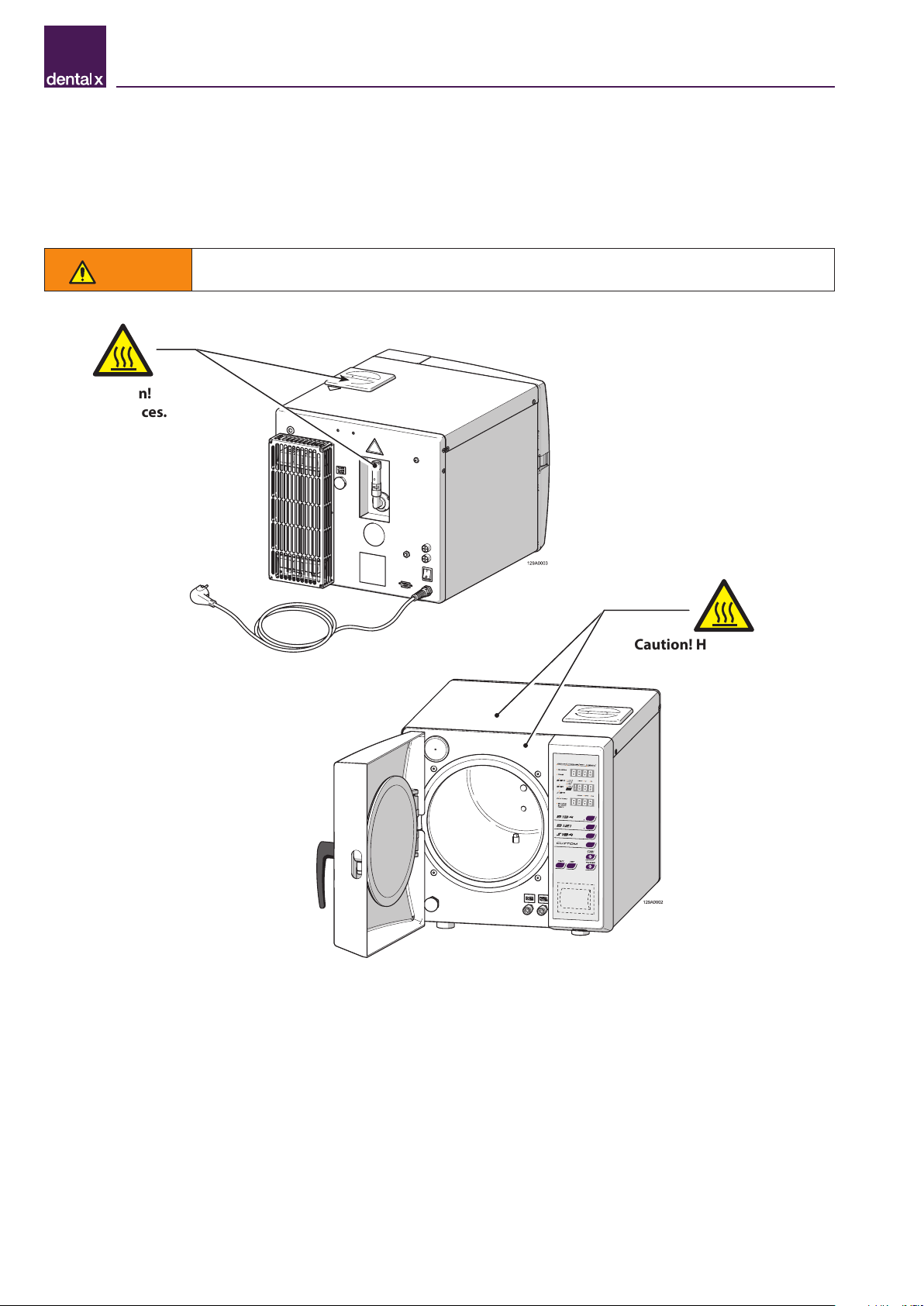
WARNING
During normal daily use of the device, residual heat risks persist in the areas marked with special
warning signs, as shown in the figure. Avoid direct contact of body parts with these surfaces.
2.5 Bacteriological risks
If the sterilization cycle is not completed, the load, the trays and their restraint system, as well as the inside of the chamber,
should always be considered as potentially contaminated until a subsequent sterilization cycle has been successfully
completed.
The water in the recovery tank should be considered as contaminated, therefore necessary precaution should be taken
when emptying the tank. Check the integrity of the drain hose before using it.
To avoid cross-contamination, wear a new pair of sterile gloves for each task. Take particular care to replace the sterile
gloves when loading or unloading instruments from the sterilization chamber and during maintenance operations.
Caution! Hot surfaces.
Caution!
Hot surfaces.

11
CHARACTERISTICS - 3
3 CHARACTERISTICS
3.1 Description of the sterilizer
The Domina Plus B is a table-top steam sterilizer designed for the sterilization of dental and medical products and equipment,
in accordance with the requirements of standard ISO EN 13060.
It consists of an airtight copper sterilization chamber accessed through a front door; it is protected by an external shock-
resistant moulded plastic body and equipped with protective devices that allow operators to use it in full safety.
The sterilization cycles are started from the operator panel on the front of the device, beside the door.
A detailed description of the units that make up the sterilizer and the components supplied is given in the following
paragraphs.
3.2 Intended use
The steam sterilizer is intended for the sterilization of reusable medical devices suited to steam sterilization in a range of
temperatures from 121 °C to 135 °C.
The types of sterilization include:
Class B sterilization
Sterilization of all wrapped and unwrapped solid material, porous products as represented by the test loads and type A and
B hollow loads.
Class S sterilization
Sterilization of products as specified by the manufacturer of the sterilizer, including unwrapped solid products and at least
one of the following:
• Porous products (fabrics)
• Small porous items
• Fluid loading or unloading products with type A and B hollows
• Single-wrapped products
• Products with multiple-layer wrapping
WARNING
Sterilizing instruments unsuitable for this process may expose the operator to risk, cause
damage to the sterilizer and compromise its safety devices.
Always check the manufacturer's label to ensure that products are suitable for sterilization.
The device is not suitable for the sterilization of liquids and flammable materials.
Do not use the device in the presence of anaesthetic or flammable gases.
NOTE The room where the device is installed should be adequately ventilated to prevent excessive
humidity.
3.3 Environmental conditions
The sterilizer is designed to operate in environments with:
• temperatures between 10°C and 40°C.
• relative humidity between 20% and 85%.
• air pressure between 750 mBar and 1050 mBar
• an altitude between 0 and 2000 meters above sea level.
Storage conditions: temperature -10°C 50°C, humidity without condensation 10-95%, atmospheric pressure 500-1060 mBar.
12
CHARACTERISTICS - 3
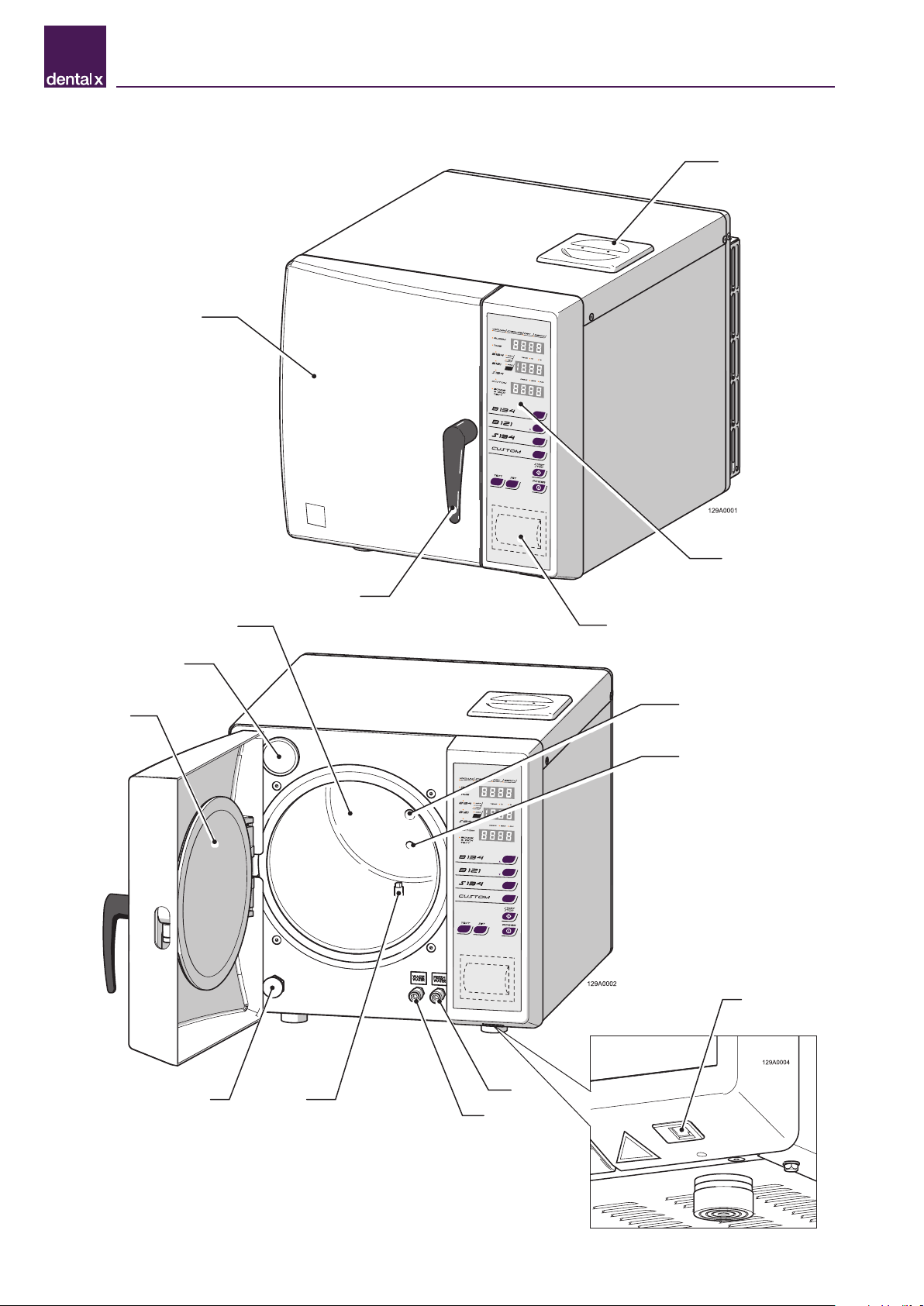
3.4 Units that make up the sterilizer
1
3
5
4
2
7
615
14
12
11
109
13
8

13
CHARACTERISTICS - 3
Position Description
1Door
2Clean demineralized water tank input
3Handle
4Operator panel
5Printer (optional)
6Steel sterilizing chamber closure disc
7Bacteriological filter
8Sterilization chamber
9Clean demineralized water tank filter
10 Drain filter
11 Quick coupling for draining contaminated water tank
12 Quick coupling for draining demineralized water tank
13 Network port for technical support service data
14 Temperature sensor
15 Safety valve connection
16 Sterilization chamber maximum pressure safety valve
17 Automatic contaminated water recovery tank outlet for the Purity device (optional)
18 Condenser protection grid
19 Power cord
20 Electrical protection fuses
21 Main switch
22 External printer port (optional)
23 Regulatory label
16
17
18
19
20
21
22
23

14
CHARACTERISTICS - 3
3.5 Components supplied with the sterilizer
Position Description
1Tray rack
2Small tray (2 pieces)
3Large tray (2 pieces)
4Rack insertion and extraction clamp
5Rubber hose with quick coupling for draining water
6Water filter extraction key
7Water filter (2 pieces)
8Bacteriological filter
9Sterilization chamber cleaning tablets (2 pieces)
10 Operator manual
11 Warranty certificate
12 Quick guide
1
2
3
4
5
7
9
10
11
6
8
12
15
CHARACTERISTICS - 3
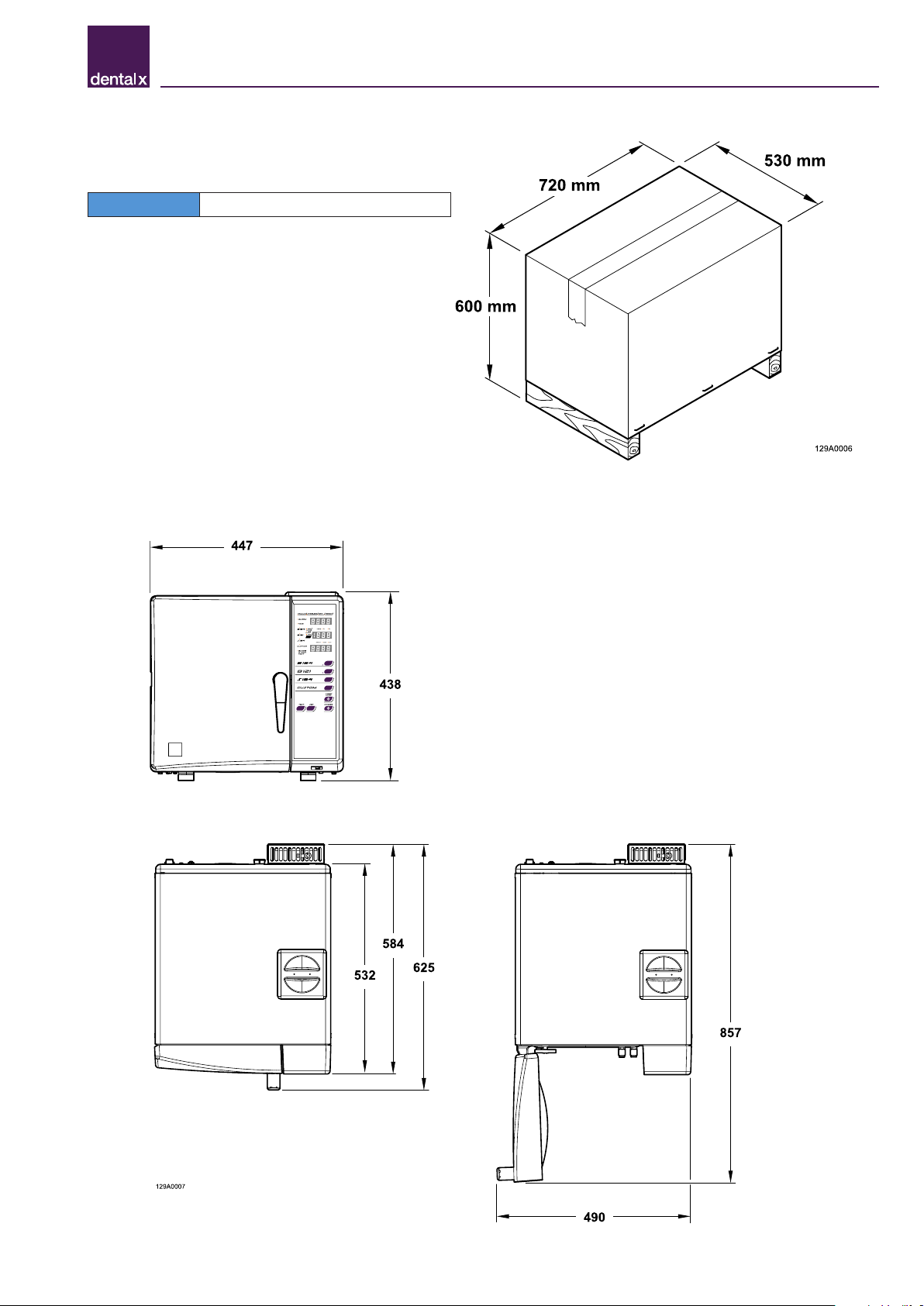
3.6 Size and weight of package
Packaging size: 720 x 600 x 530 mm (L x H x D)
Total weight of package: 55 kg
NOTE Keep the original packaging intact
3.7 Size and weight of sterilizer
STERILIZER
Net unladen weight: 45 kg
Weight with full load: 57 kg
STERILIZATION CHAMBER
Diameter: 240 mm
Depth: 384 mm
Volume: 17.5 litres
TRAYS
Usable large tray space: 315x214 mm (x 2)
Usable small tray space: 315x168 mm (x 2)
Usable volume on trays: 10 litres

16
CHARACTERISTICS - 3
3.8 Technical specifications
Chamber dimensions Ø = 240 mm D = 384 mm
Chamber volume 17.5 l
Maximum load 4 kg (solid instruments) 1.5 kg (porous instruments)
Heating time 20’ from room temperature 10’ with preheated chamber
Sterilization time From 3’ to 90’ depending on the cycle
Drying time From 3’ to 14’ depending on the cycle
External dimensions 447 x 438 x 625 mm (L x H x D)
Net weight 45 kg
Mains voltage 230 VAC
Frequency 50/60 Hz
Maximum power consumption 1920 W
Average consumption 600 W
Standby consumption 12 W (20 W printer version)
Fuses 2 x FF 10A (type 6.3 x 32 H 500V)
Clock battery Internal, not replaceable by the operator: CR2032
Automatic used-water drain rate (optional) Max 0.5 l/min, T max 70 °C
Auto-off after 30’ of inactivity at the end of the cycle
Double water tank 4 l each
“Average” water consumption for standard cycles
134°C - 121°C - 3 vacuum 584 cc - 627 cc
Vacuum pump 20 l/min - 0.96 bar
Bacteriological filter 0.3 µm at 99.97 %
IP rating (in accordance with EN 60529) IP31
Noise level 53 db
Differentiated heating system DHS
Heat transmitted to the environment at 23° C 2.16MJ
Operating cycle continuous
Pollution level 2
Transient overvoltage II
Water conductivity control H2O GOOD / H2O HARD (in reference to a value of 15
microsiemens)
Available volume on trays 10 l
Maximum chamber temperature 135°C (-0+2°C)
Safety valve intervention pressure 2.55 bar
Pressurized container conforming to Directive 2014/68/UE (PED)

17
CHARACTERISTICS - 3
3.9 Sterilizer regulatory label
The regulatory label is fixed on the back of the sterilizer and displays the CE marking together with important data for
operation, already given in the technical specifications table, and the serial number.
For convenience, the device serial number is also displayed on an adhesive label on the lower section of the internal front
panel, visible when the sterilization chamber door is open.
SERIAL NUMBER LABEL

18
CHARACTERISTICS - 3
Symbol Description
1
Symbol for manufacturer.
The data given next to this symbol identifies the
manufacturer.
NOTE: this symbol must be accompanied by the name and
address of the manufacturer.
2NSK Dental Italy S.r.l. Manufacturer's name
3Via dell’Agricoltura 21, 36016 Thiene (VI) IT Manufacturer’s address
4
Manufacturing company’s logo
5
CE marking in accordance with Dir. 93/42/EEC Medical
Devices. The CE marking certifies that the product meets
the standards applicable in the EU member states (see
declaration of conformity)
6 0051
Identification number of the notified body
Notified body IMQ: IMQ S.p.A., Via Quintiliano, 443, 20138
Milan (Italy), Identification number: 0051.
7Small steam sterilizer Explanation of use and application of the device
8MOD. Name of the device
9
Reference to catalogue Symbol on the equipment: symbol
located next to the model number (ref.to catalogue).
NOTE The manufactorer‘s catalogue number shall be after
or below the symbol adjacent to it.
10
Serial number
11 Voltage Type of power supply
12 Power Maximum power
13 Frequency Frequency
14 Fuse Type of fuses
15 Chamber capacity Chamber capacity
16 Working pressure Working pressure
17 Safety valve pressure Safety valve discharge pressure
18 Working temperature Working temperature
19
Date of manufacture.
The date given beside this symbol is the date of
manufacture.
20 MADE IN ITALY This is a merchandise mark indicating that a product is
designed, produced and packaged entirely in Italy.
19
CHARACTERISTICS - 3
21
Caution, carefully read the instructions for use before using
the device.
22
Symbol for separate waste collection of electrical and
electronic devices, in conformity with Directive 2012/19/EU
(WEEE/RAEE).

20
INSTALLATION - 4
4 INSTALLATION
4.1 Unpacking and transportation
The packaging of the sterilizer consists of a wooden pallet on which the sterilizer is placed, with adequate protective padding
and a corrugated cardboard casing fixed to the pallet with metal staples.
Place the package on a level surface free from clutter to facilitate easy opening and safe extraction of the sterilizer.
Remove the staples holding the casing to the pallet.
Remove the corner and edge
protection from the sterilizer.
Lift the sterilizer and position it in
the place of installation.
CAUTION
Lifting, transporting and positioning the sterilizer in the place of installation should be
performed by two people.
Remove the cardboard casing.
Table of contents
Other dental X Laboratory Equipment manuals
Popular Laboratory Equipment manuals by other brands

Belden
Belden HIRSCHMANN RPI-P1-4PoE installation manual

Koehler
Koehler K1223 Series Operation and instruction manual

Globe Scientific
Globe Scientific GCM-12 quick start guide

Getinge
Getinge 86 SERIES Technical manual

CORNING
CORNING Everon 6000 user manual

Biocomp
Biocomp GRADIENT MASTER 108 operating manual












