Breas HA 01 User manual

1Intended Use of the Breas HA 01 Humidifier
Breas HA 01 Humidifier User Manual
Doc. 004221 En T-2
1 Intended Use of the Breas HA 01 Humidifier
The humidifier is intended to humidify the patient air.
User Manual for
Breas HA 01 Humidifier
Read this manual thoroughly so that you completely understand how
the humidifier is operated and maintained before taking it into use, to
ensure correct usage, maximum performance and serviceability.
Breas Medical AB reserves the right to make changes to this product
without any prior notification.

2 Intended Use of the Breas HA 01 Humidifier
Breas HA 01 Humidifier User Manual Doc. 004221 En T-2
1.1 Indications for Use
The humidifier can only be used together with Breas breathing therapy equip-
ment, such as iSleep or Vivo.
It must always be prescribed by a licensed physician.
1.2 About this Manual
Icons
In this manual, icons are used to highlight specific information. The meaning of
each icon is explained in the table below.
For complete instructions how to operate the humdifier, refer to the Breas breathing
therapy equipment manual.
ICON EXPLANATION
War ning!
Risk of death and serious personal injury.
Caution!
Risk of minor or moderate injury. Risk of equipment damage,
loss of data, extra work, or unexpected results.
Note
Information that may be valuable but is not of critical impor-
tance, tips.
Reference
Reference to other manuals with additional information on a
specific topic.

3Safety Information
Breas HA 01 Humidifier User Manual
Doc. 004221 En T-2
2 Safety Information
2.1 General User Precautions
• The humidifier should be located below the patient to prevent personal
injury from accidental spillage.
• Periodically check for moisture in the patient circuit. When present,
remove the moisture. Before attempting to dry the circuit, disconnect it
from the humidifier to ensure no water back-flow into the humidifier. The
frequency at which these checks must be performed will depend on the
patient’s own condition and the device used. This should be assessed on an
individual basis in accordance with the patient’s needs.
• If the condensation in the patient circuit is excessive, the use of a heated
humidifier may require the installation of a water trap in the circuit. The
water trap prevents any condensated water in the patient circuit from run-
ning into the patient airways and causing personal injury
• The humidifier must only be used:
– for the intended treatment in accordance with this manual and with the
instructions given by the responsible clinical personnel;
– in accordance with the operating conditions specified in this manual;
– in original and unmodified shape and only with accessories specified or
approved by Breas Medical AB.
• Do not use the humidifier and contact your responsible care provider for
an inspection in the event of suspected damage to the device.
• Handle the humidifier with care.
• The humidifier shall be disconnected from the Breas breathing therapy
equipment during transportation.
• The Breas breathing therapy equipment shall not be placed in the bag with
the humidifier attached.
4 Safety Information
Breas HA 01 Humidifier User Manual Doc. 004221 En T-2
2.2 Electrical Safety
2.3 Environmental Conditions
2.4 Cleaning and Maintenance
• When handling the humidifier, disconnect the Breas breathing therapy
equipment from any power source.
• Do not connect cables to the Breas breathing therapy equipment when
there is water in the humidifier.
• The performance of the humidifier may deteriorate at:
– ambient temperatures below 5°C (41°F) and above 40°C (104°F).
– ambient relative humidity below 10% RH (room humidity) and above 95%
RH.
– atmosphere pressure below 700 mbar and above 1060 mbar.
• The humidifier, any accessories and all replaced parts must be disposed of
in accordance with the local environmental regulations regarding the dis-
posal of used equipment and waste.
• The humidifier shall be cleaned and maintained in accordance with this
manual.
• Do not attempt to autoclave or sterilise the humidifier.
• Recommended usage period of the humidifier: 12 months.
Maximum usage period: 24 months.
• Do not under any circumstances attempt to service or repair the humidifier
yourself. If you do so, the manufacturer will no longer be responsible for
the performance and safety of the humidifier.

5Product Description
Breas HA 01 Humidifier User Manual
Doc. 004221 En T-2
3 Product Description
3.1 Equipment Designation and Safety Label
NO. COMPONENT FUNCTION
1 Humidifier lid Sealing the humidifier.
2 Internal pipe To prevent water spillage into the Breas
breathing therapy equipment.
3 Swivel Patient tubing connector.
4 Max Marking the maximum level of water.
5 Water container Contains and heats water.

6 Using the Humidifier
Breas HA 01 Humidifier User Manual Doc. 004221 En T-2
4 Using the Humidifier
Adding Water to the Humidifier
SYMBOL EXPLANATION
Model designation
Serial number (the last six alphanumeric characters)
Class II electrical equipment; dual isolation
Body floating (IEC 60601-1 Type BF, Isolated Applied
Part)
Read the user manual thoroughly before connecting the
humidifier to the patient.
CE marking applies in accordance with the directive
MDD 93/42/EEC.
The unit may be returned to Breas Medical AB for scrap-
ping and should not be discarded with the household
waste.
• Never add or pour out water from the humidifier when attached to the
Breas breathing therapy equipment.
• Prevent water from entering the Breas breathing therapy equipment.
• Always turn off the Breas breathing therapy equipment and disconnect the
mains supply before removing the humidifier.
• Do not use the humidifier if the internal pipe in the water container is miss-
ing.
• Do not overfill the humidifier.
• If there is water outside of the humidifier after filling, dry the humidifier
using a lint-free cloth before reconnecting it to the Breas breathing therapy
equipment.
7Using the Humidifier
Breas HA 01 Humidifier User Manual
Doc. 004221 En T-2
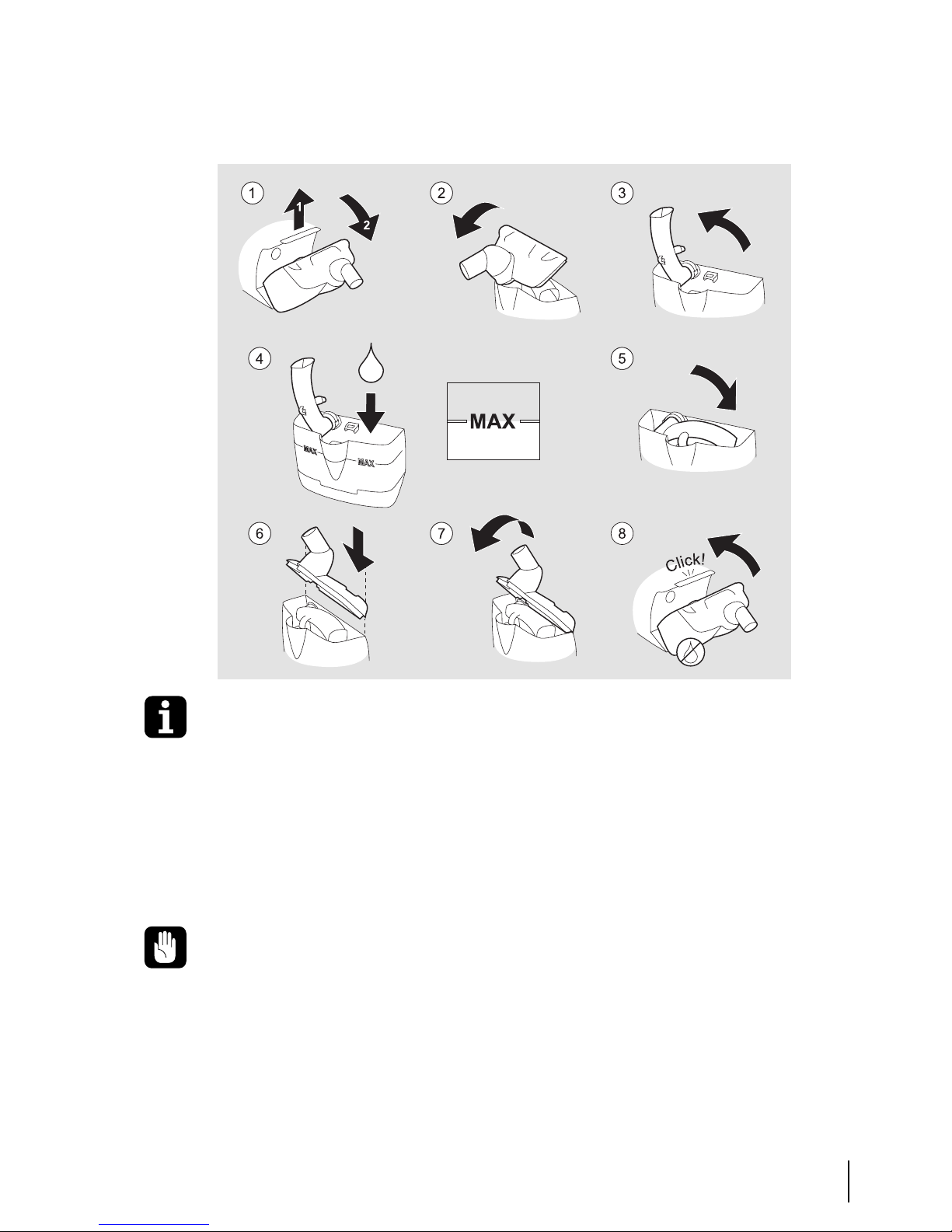
Follow the instruction in the illustration below. Use the same procedure when
emptying water from the humidifier.
Cleaning the Humidifier
• Clean with hot water and a mild detergent or in a dishwasher without dish-
washer detergent at max 70°C. Rinse carefully and allow to dry.
• The humidifier can be disinfected with Virkon
®
or Lysetol.
®
4.1 Disposal
The humidifier, any accessories and all replaced parts must be disposed of and
recycled in accordance with the local environmental regulations regarding the
disposal of used equipment and waste.
Add distilled water or boiled, chilled tap water to the humidifier until it reaches
the marking “Max” on the humidifier. A full humidifier contains approximately
400 ml. Do not overfill the humidifier.
Do not attempt to autoclave the humidifier. Exposure to temperatures
above 100°C causes irreparable damage.

8 Technical Specifications
Breas HA 01 Humidifier User Manual Doc. 004221 En T-2
5 Technical Specifications
5.1 Data
POWER SUPPLIES SPECIFICATION
Power supply 12-30 V DC, max 40 W.
ENVIRONMENTAL
CONDITIONS SPECIFICATION
Operating temperature
range
5 to 40°C (41 to 104°F)
Storage and transport tem-
perature
-20 to +60°C (-4 to +140°F)
Ambient pressure range 700 to 1060 mbar
Humidity 10% to 95%, non-condensing
DIMENSIONS SPECIFICATIONS
W
×
H
×
D 148
×
155
×
98 mm
Weight 260 g
Air outlet 22 mm conical standard connector
PERFORMANCE SPECIFICATIONS
Gas leakage at max. operat-
ing pressure
< 5 ml/min

9Technical Specifications
Breas HA 01 Humidifier User Manual
Doc. 004221 En T-2
The diagram below shows the output of the humidifier at the patient end of the
tube:
The warm-up time for the delivered gas temperature to reach the set tempera-
ture from a starting temperature of 23 ±2°C is less than 60 minutes when oper-
ated according to Breas instructions.
: Setting 3
: Setting 6
: Setting 9
Flow ( l/min )
Output of H
2
O ( mg/l )

10 Technical Specifications
Breas HA 01 Humidifier User Manual Doc. 004221 En T-2
5.2 Compliance of Standards
STANDARD SPECIFICATIONS
IEC 60601-1 (1988)
A1 (1991)
A2 (1995)
Medical electrical equipment - Part 1: General
requirements for safety.
IEC 60601-1-1 (2000) Medical electrical equipment - Part 1-1: Gen-
eral requirements for safety - Collateral stand-
ard: Safety requirements for medical electrical
systems.
IEC 60601-1-2 (2001) Medical electrical equipment - Part 1-2: Gen-
eral requirements for safety - Collateral stand-
ard: Electromagnetic compatibility -
Requirements and tests.
IEC 60601-1-4 (2000) Medical electrical equipment - Part 1-4: Gen-
eral requirements for safety - Collateral Stand-
ard: Programmable electrical medical systems.
IEC 60601-1-6 (2004) Medical electrical equipment - Part 1-6: Gen-
eral requirements for safety - Collateral stand-
ard: Usability.
ISO 8185 (1997)
C1(2001)
Humidifiers for medical use - General require-
ments for humidification systems.
CLASSIFICATIONS SPECIFICATIONS
Class IIa Classification according to the European
Medical Device Directive 93/42/EEC.
Class II FDA classification
Class II (IEC 60601-1) Class
II, Type BF
Electrical equipment with dual isolation and
body floating (isolated) applied part according
to IEC 60601-1.
IPX1 Degree of protection provided by enclosure.
The humidifier and its packaging do not contain any natural rubber latex.

11Notes
Breas HA 01 Humidifier User Manual
Doc. 004221 En T-2
6Notes
Table of contents
Other Breas Humidifier manuals