Charder HM80M User manual
1
USER MANUAL
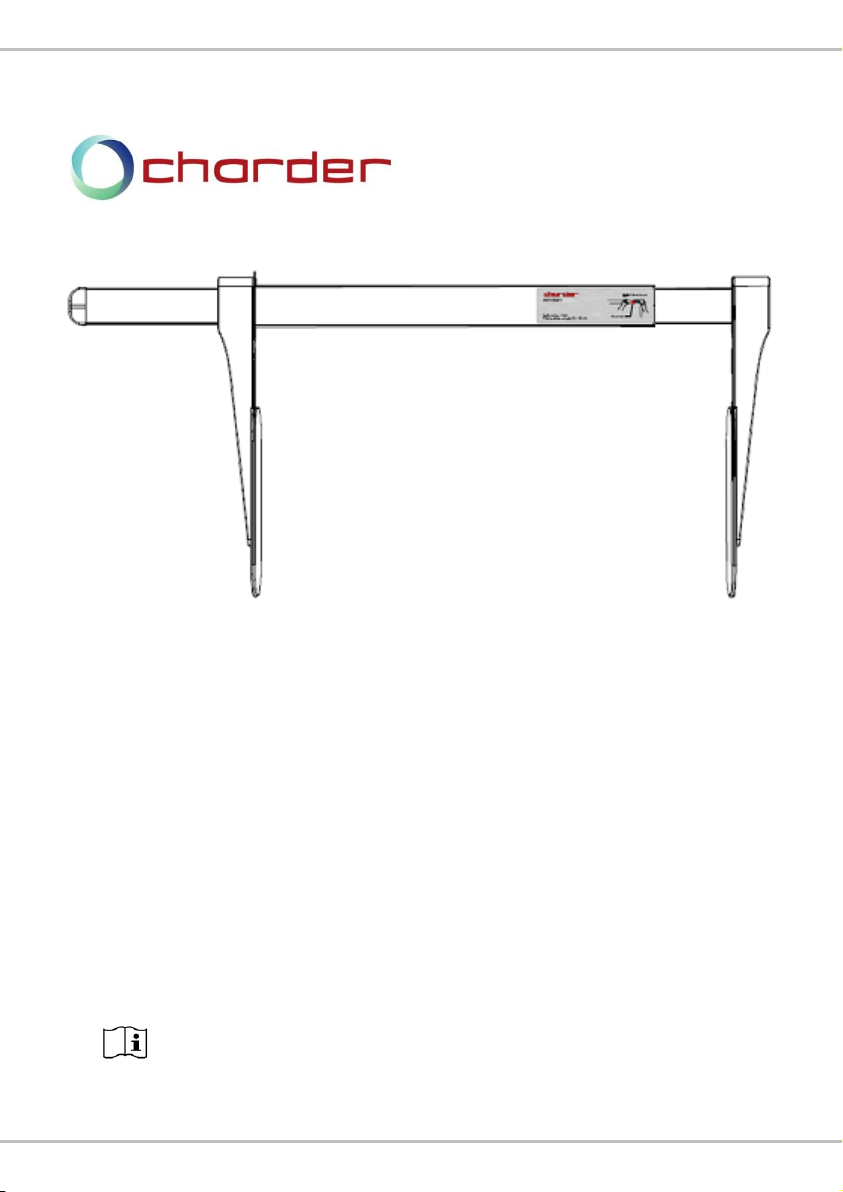
HM80M
Portable Height Meter
Please keep the instruction manual at hand all the time for future reference.

2
Explanation of Graphic Symbols on Label/Packaging
Caution, consult
accompanying documents
before use
Authorized
representative in the
European Community
Manufacturer of medical
device
Indicates that device
conforms to Regulation
(EU) 2017/745. Four
digit number refers to
Notified Body.
Manufacturing year of
medical device
Indicates that device is
a medical device
Device catalogue number
Serial number
Manufacturer's batch or lot
number
Unique Device Identifier
Carefully read user manual
before installation and usage,
and follow instructions for
use.

3
Copyright Notice
Charder Electronic Co., Ltd.
No.103, Guozhong Rd., Dali Dist., Taichung City 41262 Taiwan
Tel: +886-4-2406 3766
Fax: +886-4-2406 5612
Website: www.chardermedical.com E-mail: info_cec@charder.com.tw
Copyright© Charder Electronic Co., Ltd. All rights reserved.
This user manual is protected by international copyright law. All content is
licensed, and usage is subject to written authorization from Charder
Electronic Co., Ltd. (hereinafter Charder) Charder is not liable for any
damage caused by a failure to adhere to requirements stated in this
manual. Charder reserves the right to correct misprints in the manual
without prior notice, and modify the exterior of the device for quality
purposes without customer consent.
Charder Electronic Co., Ltd.
No. 103, Guozhong Rd., Dali Dist.,
Taichung City, 412 Taiwan

4
CONTENTS
I. Safety Notes .......................................................................... 5
A. General Information .............................................................5
II. Installation........................................................................... 7
A. Attaching head stopper.........................................................7
B. Assembly with Infant Scale (removable measurement tray) ......7
C. Assembly with MS5900 Infant Scale .......................................8
D. Assembly with MS2400 Infant Scale ..................................... 11
III. Using Device ......................................................................13
A. Performing measurement.................................................... 13
IV. Product Specifications ........................................................14
A. Device Information ............................................................ 14
V. Declaration of Conformity ....................................................16

5
I. Safety Notes
A. General Information
Thank you for choosing this Charder Medical device. It is designed to be
easy and straightforward to operate, but if you encounter any problems
not addressed in this manual, please contact your local Charder service
partner.
Before beginning operation of the device, please read this user manual
carefully, and keep it in a safe place for reference. It contains important
instructions regarding installation, proper usage, and maintenance.
Intended Use
This device is intended to measure the height of subjects, for diagnosis of
height-related issues by professionals.
General Handling
Device should be placed on stable, flat, solid, non-slippery surface.
Ensure all parts are properly locked and tightened before operating
the device.
Measurement accuracy requires the subject's feet, back, and head to
be straightly aligned. Please note that height can vary throughout
the day
Safety Instructions
Before putting device into use, please read this user manual carefully. It
contains important instructions for installation, usage, and maintenance
of device.
The manufacturer shall not be liable for damages caused by failure to
heed the following instructions:
Expected service life: 5 years.
Improper installation will render the warranty null and void.
Observe permissible ambient temperatures for use
Cleaning
Device surface should be cleaned using alcohol-based wipes.

6
Warranty/Liability
If Charder is responsible for a fault or defect present upon receipt of
the unit, Charder shall either repair the fault, or supply a
replacement unit. Should the repairs or replacement delivery fail,
statutory provisions shall be valid. The period of warranty shall be
two years, beginning on the date of purchase. Please retain your
receipt as proof of purchase.
No responsibility shall be accepted for damage caused through any
of the following reasons: unsuitable or improper storage or use,
incorrect installation or commissioning by the owner or third parties,
natural wear and tear, changes or modifications, incorrect or
negligent handling, chemical, electrochemical, or electrical
interference, unless damage is attributable to negligence on the part
of Charder.
This device does not contain any user-maintained parts. All
maintenance, technical inspections, and repairs should be conducted
by an authorized Charder service partner, using original Charder
accessories and spare parts. Charder is not liable for any damages
arising from improper maintenance or usage. Dismantlement of the
device will void the warranty.
Incident Reporting
Any serious incident that has occurred in relation to the device
should be reported to the manufacturer, EU representative (if device
is used in EU member state), and competent authority of
user/subject's member state.
7
II. Installation
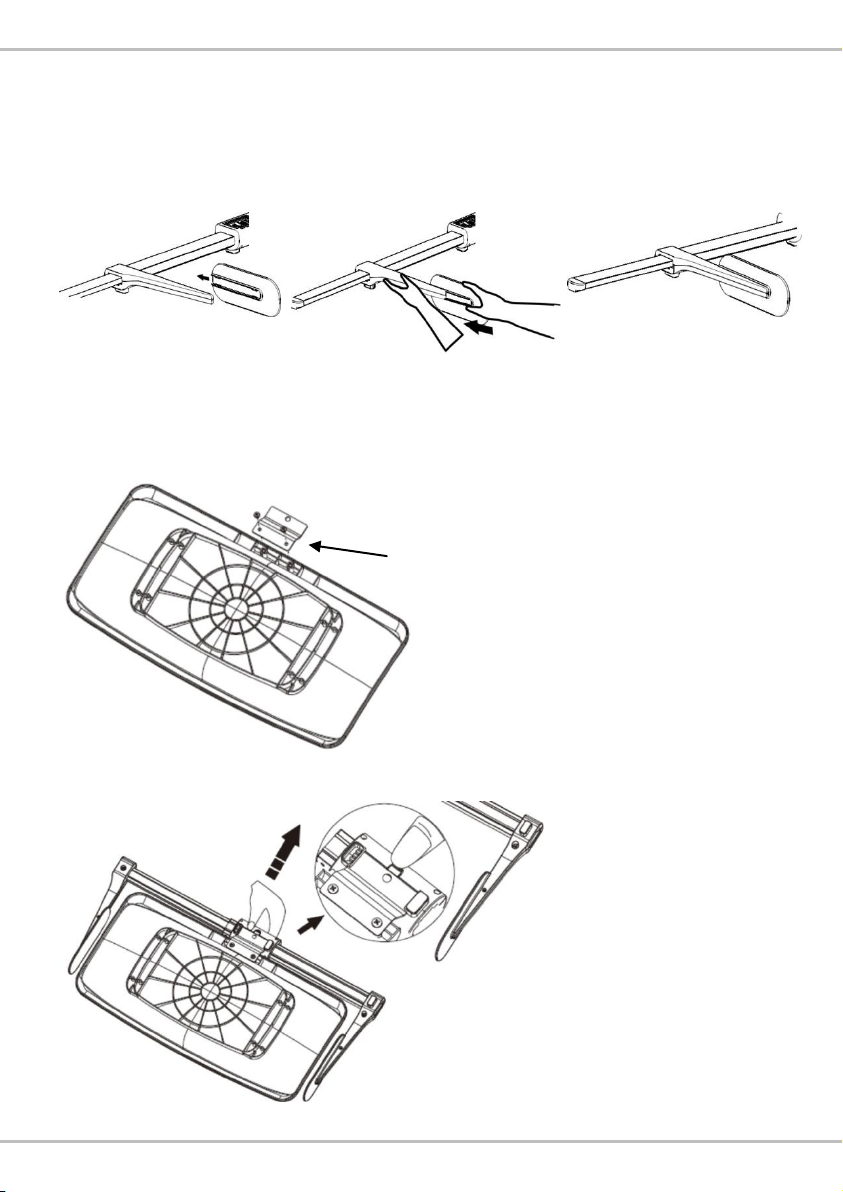
A. Attaching head stopper
If head stopper is loose, please assemble as seen below:
1.
2.
3.
B. Assembly with Infant Scale (removable measurement tray)
1. Attach bracket to tray using bracket screws*2
2. Slide HM80M onto bracket, and press buckle to secure
SS-5611 bracket

8
C. Assembly with MS5900 Infant Scale
1. Remove bracket holder cover.
2. Attach bracket to device with two screws.
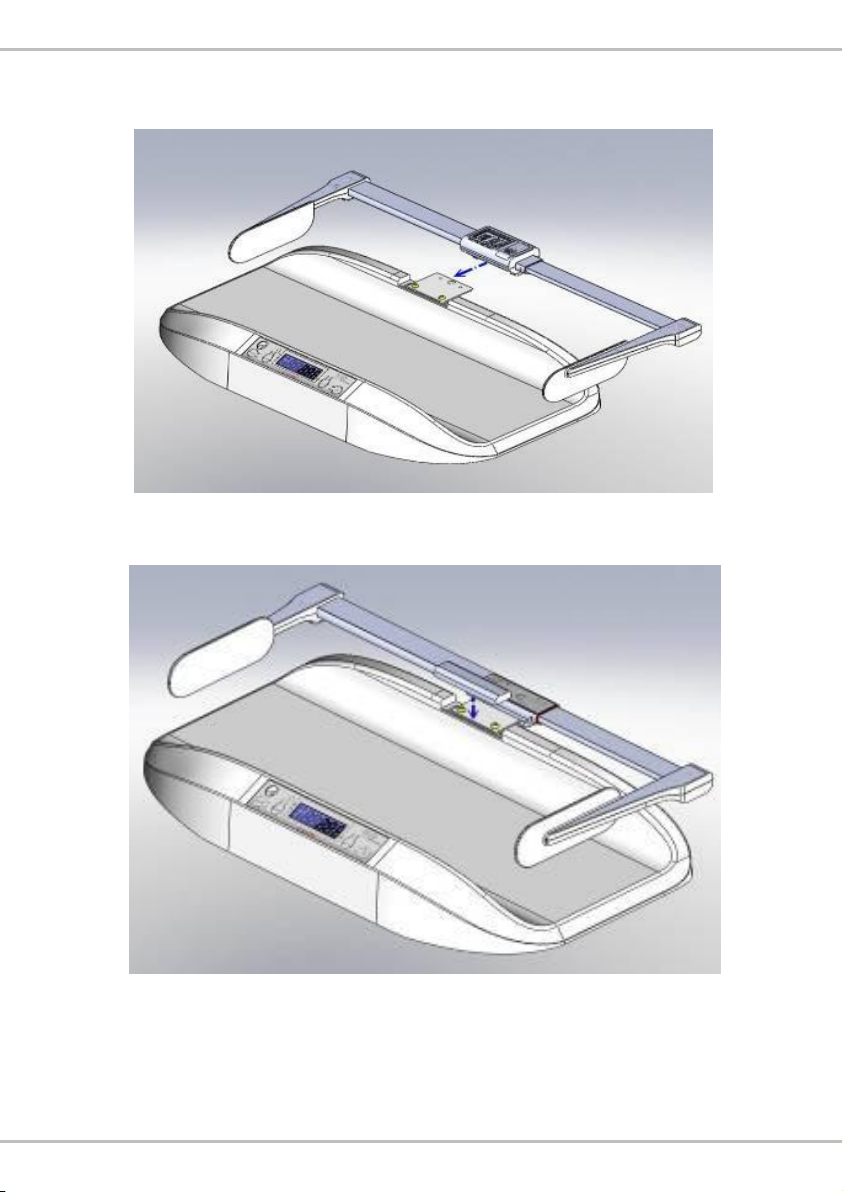
9
3. Attach height rod to bracket carefully until a click noise is heard.
(HM80D used as example, same procedure for HM80M)
4. Install bracket holder cover.

10
5. Disassembling height rod
Locate buckle at bottom of height rod. Press down on buckle and slid out
height rod.
11
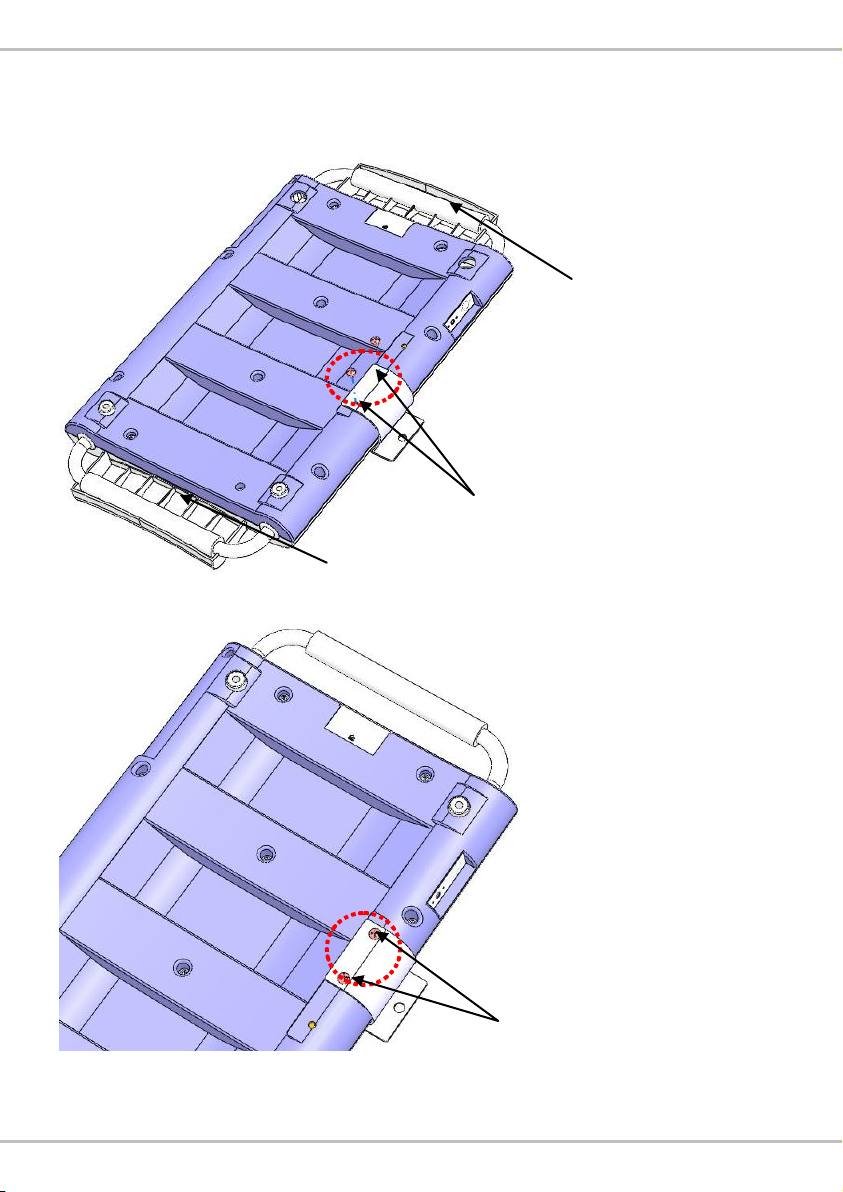
D. Assembly with MS2400 Infant Scale
1. Attach bracket to device, and fasten screws using screwdriver.
Version with rubber head pad
Version without rubber head pad
2. Connect height measure attachment (HM80D or HM80M) to bracket. A
clicking noise will be heard.
Attach bracket here
rubber head pad
rubber head pad
Attach bracket here

12
3. To detach height measure, find the latch located at the rear of the
attachment. Press down on the buckle, and gently remove the
attachment from bracket.

13
III. Using Device
A. Performing measurement
1. Lay infant on flat platform, with head touching head stopper. Straighten
infant’s feet.
2. Use right hand to push foot stopper until it touches soles of infant's feet.
Read measurement result.
14
IV. Product Specifications
A. Device Information
Model
HM80M
Height
Measurement
Range
35-80 cm
13 3/4-31 1/2 in
Graduation
1 mm
1/16 in
Accuracy
± 10 mm
Dimensions
Overall
870(W) x 290(D) x 70(H) mm
Device
Weight
0.5 kg
Operation Temperature &
Humidity
5℃~35℃
Optional Accessories
Bracket set for installation on
compatible Charder Infant Scale
Standard Accessories
User manual x1

15
Notes
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
_______________________________________________________
16
V. Declaration of Conformity
This product has been manufactured in accordance with the harmonized
European standards, following the provisions of the below stated
directives:
93/42/EEC as amended by 2007/47/EC
Medical Device Directive
Please see separate document showing on sticker of device for above CE
marking.
Authorized EU Representative:
Manufactured by:
Charder Electronic Co., Ltd.
No.103, Guozhong Rd., Dali Dist.,
Taichung City, 412 Taiwan (R.O.C.)
CD-IN-1576 REV XXX 07/2020
This manual suits for next models
1
Table of contents
Other Charder Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Datascope
Datascope Accutorr Plus Service manual

Stryker Medical
Stryker Medical Venture Series Maintenance manual

Natural Ozone
Natural Ozone OXY-90 Safety & instruction manual
Lojer
Lojer SALLI Series operating instructions
RhythMedix
RhythMedix RhythmStar Operator's manual

GE
GE Solar 8000M/i Service manual