Avedro Mosaic System User manual
Mosaic Operator’s Manual Rev F
ML-00023
Avedro, Inc.
Mosaic® System
Operator’s Manual
Copyright 2019. All Rights Reserved.
Printed in U.S.A.
Mosaic Operator’s Manual Rev F
ML-00023
Patents, Trademarks, Copyrights
The Mosaic System may be covered by one or more patent applications
issued or pending in the United States and worldwide.
“Mosaic” and the Avedro logo design are registered trademarks or
trademarks of Avedro, Inc. All software and documentation are subject to
Avedro, Inc. copyrights. All rights reserved 2019.
Microsoft and Windows are registered trademarks and trademarks,
respectively, of Microsoft Corporation. Any other trademarks or service
marks contained within this manual are the property of their respective
owners.
For more information, contact: Your Local Avedro-authorized
distributor
Avedro, Inc.
201 Jones Road
Waltham, MA 02451
Authorized Representative
EMERGO EUROPE
Prinsessegracht 20
2514 AP, The Hague
The Netherlands
Phone: +31.70.345.8570
Fax: +31.70.346.7299
0086
Mosaic Operator’s Manual Rev F
ML-00023
Table of Contents
1Foreword.................................................................................................................................... 1
Intended Use of Manual ................................................................................................................1
Intended Use / Indications for Use..........................................................................................1
Essential Requirements.................................................................................................................1
Design Change Disclaimer...........................................................................................................1
Reproduction Disclaimer.............................................................................................................2
User Operation Assistance Statement .................................................................................2
Contraindications, Warnings, and Cautions.......................................................................2
1.7.1 Contraindications................................................................................................................2
1.7.2 Warnings and Cautions...................................................................................................2
1.7.3 Electrical Safety Warnings............................................................................................3
1.7.4 Radiation Safety Warnings...........................................................................................5
Patient Safety....................................................................................................................................6
Additional Safety Considerations ...........................................................................................6
FCC Compliance Notice...............................................................................................................6
2Introduction.............................................................................................................................. 8
System Overview ............................................................................................................................8
2.1.1 Major Components.............................................................................................................9
2.1.2 Disposables .........................................................................................................................10
2.1.3 Mosaic System Labels .....................................................................................................11
2.1.4 Frequently Used Functions ........................................................................................ 12
3System Operation.................................................................................................................. 13
Touchpad/Keyboard Use.......................................................................................................... 13
UV Energy (Dose)......................................................................................................................... 14
4System Set Up ........................................................................................................................ 15
Important Steps before Turning on the System ........................................................... 15
Preparing the System.................................................................................................................. 15
Powering Up .................................................................................................................................... 15
Startup Screen................................................................................................................................ 16
Setting Optics Head Height ..................................................................................................... 17
Proximity Warning........................................................................................................................ 18
5Treatment Procedure ...........................................................................................................19
Patient Central ................................................................................................................................ 19
5.1.1 Entering a New Patient.................................................................................................. 19
5.1.2 Importing Patient Topographies.............................................................................20
Designing a Patient Treatment .............................................................................................20
5.2.1 Verifying Pupil Location..............................................................................................20
5.2.2 Overview of the Treatment Designer................................................................... 21
5.2.3 Treatment Type Options ........................................................................................... 22
5.2.4 Designing a KXL Treatment..................................................................................... 22
5.2.5 Designing a PiXL or CuRV Treatment................................................................. 23
5.2.6 Multi-Shape Treatments............................................................................................. 25
5.2.7 Settings............................................................................................................................... 25
5.2.8 Adjusting Power (Irradiance) and UV Mode ................................................... 27
5.2.9 Confirm Treatment Design....................................................................................... 27
Treatment Activation Card ..................................................................................................... 28
5.3.1 Multi-use Treatment Activation Cards.................................................................29

Mosaic Operator’s Manual Rev F
ML-00023
Set the Riboflavin Induction Time ....................................................................................... 29
5.4.1 Mosaic Induction Timer ...............................................................................................30
5.4.2 External Induction Timer ...........................................................................................30
Prepare the Patient .....................................................................................................................30
Apply Riboflavin............................................................................................................................. 31
Coarse Alignment of Laser Crosshairs .............................................................................. 32
Manual Axis Alignment Mode ................................................................................................ 33
Auto Alignment............................................................................................................................. 33
5.9.1 Unsuccessful Auto Alignment.................................................................................. 35
Begin UV Treatment................................................................................................................... 37
Treatment Complete ..................................................................................................................38
Treatment Incomplete ...............................................................................................................39
Power Down the System..........................................................................................................39
6Device Settings ......................................................................................................................41
Using the Device Settings Menu............................................................................................ 41
6.1.1 Advanced Settings........................................................................................................... 41
6.1.2 Transfer Data to USB..................................................................................................... 41
6.1.3 Edit Default Treatment Parameters......................................................................42
6.1.4 Patient Prescreen Test.................................................................................................43
6.1.5 Demo Mode .......................................................................................................................47
7Maintenance / Service ........................................................................................................48
Installation Policy..........................................................................................................................48
Customer Maintenance..............................................................................................................48
Warranty Information.................................................................................................................48
Per Patient Disposables ............................................................................................................48
Trouble Shooting..........................................................................................................................49
Directions for Disinfection .......................................................................................................49
Cleaning the System...................................................................................................................49
Cleaning the Aperture................................................................................................................50
Moving the System......................................................................................................................50
Storing the System....................................................................................................................... 51
Software............................................................................................................................................. 51
Identifying Risks Associated with Disposing of Waste Products........................ 52
Performing a Visual Check ...................................................................................................... 52
8Equipment Classification.................................................................................................... 53
EMC Requirements......................................................................................................................54
9Symbol Library ...................................................................................................................... 59
10 Specifications ........................................................................................................................ 62

Mosaic Operator’s Manual Rev F
ML-00023
Table of Figures
Figure 2-1. Overview Illustration of Mosaic System.............................................................................. 9
Figure 2-2. System Overview with Callouts ............................................................................................10
Figure 2-3. Mosaic Label .................................................................................................................................... 11
Figure 2-4. UV Safety Label ............................................................................................................................. 11
Figure 2-5. Laser Classification Label .......................................................................................................... 11
Figure 2-6. Do Not Sit/Step On Label......................................................................................................... 11
Figure 4-1. Startup Screen................................................................................................................................16
Figure 4-2. Lift Buttons...................................................................................................................................... 17
Figure 4-3. Optics Head .................................................................................................................................... 17
Figure 4-4. Proximity Warning: Move lift up...........................................................................................18
Figure 5-1. Import/Enter Patient Data Screen........................................................................................19
Figure 5-2. Verifying Pupil Location...........................................................................................................20
Figure 5-3. Treatment Designer Layout.................................................................................................... 21
Figure 5-4. Create KXL Treatment Design .............................................................................................22
Figure 5-5. Adjust Energy for KXL Treatment......................................................................................23
Figure 5-6. Treatment Shape Selection....................................................................................................24
Figure 5-7. Selecting Energy Dose and Centering Shape...............................................................24
Figure 5-8. Resulting Treatment Pattern.................................................................................................25
Figure 5-9. Settings Menu ...............................................................................................................................26
Figure 5-10. Settings for Map Options ......................................................................................................26
Figure 5-11. Power and Pulse Settings ....................................................................................................... 27
Figure 5-12. Confirm Treatment Design Window ................................................................................28
Figure 5-13. Activation Card Not Detected ............................................................................................28
Figure 5-14. Final Treatment...........................................................................................................................29
Figure 5-15. Induction Timer Options ........................................................................................................29
Figure 5-16. Two-Part Induction Timer .................................................................................................... 30
Figure 5-17. Apply Riboflavin & Start Timer Screen ............................................................................ 31
Figure 5-18. Coarse Alignment with Lasers ............................................................................................32
Figure 5-19. Manual Axis Alignment Mode..............................................................................................33
Figure 5-20. Alignment in Progress............................................................................................................34
Figure 5-21. Alignment Complete ................................................................................................................34
Figure 5-22. Z-Alignment Error ....................................................................................................................35
Figure 5-23. Iris Registration Failure ..........................................................................................................35
Figure 5-24. Restart Auto Alignment or Manual Alignment Option..........................................36
Figure 5-25. Toggling between Manual Axis Alignment & Alignment Lasers ......................36
Figure 5-26. Begin UV Treatment ...............................................................................................................37
Figure 5-27. Treatment in Progress............................................................................................................37
Figure 5-28. Pausing UV Treatment...........................................................................................................38
Figure 5-29. Treatment Complete Screen ..............................................................................................38
Figure 5-30. Treatment Incomplete Annotation..................................................................................39
Figure 5-31. Power Down System............................................................................................................... 40

Mosaic Operator’s Manual Rev F
ML-00023
Figure 6-1. Device Settings Menu..................................................................................................................41
Figure 6-2. Device Settings Transfer to USB.........................................................................................42
Figure 6-3. Edit Default Treatment Parameters (Continuous & Pulsed).................................42
Figure 6-4. Insert flash drive ..........................................................................................................................43
Figure 6-5. Enter IOL Information..............................................................................................................43
Figure 6-6. Alignment Step ........................................................................................................................... 44
Figure 6-7. Screening Step Process .......................................................................................................... 44
Figure 6-8. First Attempt Fail........................................................................................................................45
Figure 6-9. Failed Prescreen Assessment Due to Eye Tracking Errors ...................................45
Figure 6-10. Confirm Patient Prescreen Results...................................................................................46
Figure 6-11. Prescreen Test Pass...................................................................................................................46
Figure 7-1. Moving Position of the Mosaic System............................................................................. 50
Figure 7-2. Example Error Message Box................................................................................................... 51

AVEDRO | 1of 62
ML-00023
1Foreword
Intended Use of Manual
This manual is designed to serve operators of the Avedro, Inc. Mosaic
System. All operating instructions, product illustrations, screen graphics,
troubleshooting/error messages, and other relevant information are
contained in this manual. It is the operator’s responsibility to ensure that
all safety instructions in this manual are strictly followed.
Intended Use / Indications for Use
The Mosaic System delivers a uniform, metered dose of UVA light to a
targeted treatment area for the intended use of illuminating the cornea
during corneal cross-linking procedures.
Essential Requirements
The Mosaic System must maintain the programmed intensity and spatial
characteristics of the emitted UVA light while in treatment mode.
Design Change Disclaimer
•Due to design changes and product improvements, information
in this manual is subject to change without notice. Avedro, Inc.
(hereafter called “Avedro”) reserves the right to change
product design at any time without notice, which may
subsequently affect the contents of this manual.
•Avedro assumes no responsibility for any errors that may
appear in this manual. Avedro will make every reasonable effort
to ensure that this manual is up to date and corresponds with
the shipped Mosaic System.
•The computer display screens depicted in this manual are
representative only. Depending on the software version of the
System, minor differences may appear between the actual
computer displays and those shown in this manual.
•All patient data appearing in this document, including the
sample screen graphics, are fictitious and representative only.
No patient’s confidentiality has been violated, with or without
permission.

Mosaic Operator’s Manual Rev F| Page
2
ML-00023
Reproduction Disclaimer
Neither this manual nor any part of it may be reproduced, photocopied,
or electronically transmitted in any way without the advanced written
permission of Avedro.
User Operation Assistance Statement
Should you experience any difficulty in running your Mosaic System,
please contact your local Avedro authorized representative.
Contraindications, Warnings, and Cautions
1.7.1 Contraindications
This section describes situations in which the device should not be used
because the risk of use clearly outweighs any possible benefit.
Conditions that may contraindicate the use of the device include:
•Corneal thickness, with epithelium of less than < 375 microns
•Corneal melting disorders
•Aphakic patients
•Pseudophakic patients without UV blocking lens implanted
•Pregnant and nursing women
•Children
1.7.2 Warnings and Cautions
In this manual, “Warning” is defined as: a statement that alerts the user
to the possibility of injury, death, or other serious adverse reactions
associated with the use or misuse of the device. Physicians should
evaluate the potential benefits in patients with the following conditions:
•Herpes simplex, herpes zoster keratitis, recurrent corneal
erosion, corneal dystrophy
•Epithelial healing disorders
In this manual, “Caution” is defined as: a statement that alerts the user
to the possibility of a problem with the device associated with its use
or misuse. Such problems include device malfunction, device failure,
damage to the device or damage to other property. The caution
statement includes the precaution that should be taken to avoid the
hazard.
AVEDRO | 3of 62
ML-00023
1.7.3 Electrical Safety Warnings
•This equipment requires special precautions regarding
electromagnetic compatibility (EMC). Installation and use
should be carried out according to the EMC information
provided in this manual.
•Portable and mobile RF communications equipment can affect
medical electrical equipment such as the Mosaic System.
For Equipment Classifications, please refer to Chapter 7: Equipment
Classifications
WARNING: Any repair or service must be carried out by
Avedro trained personnel only.
WARNING: Do NOT modify this equipment without
authorization of the manufacturer.
WARNING: To avoid the risk of shock this equipment must
only be connected to a supply mains with protective earth.
To separate System connection to mains, grasp the power
cord plug and pull it from outlet to disconnect.
The System is designed for continuous operation using the
external connector.
WARNING: This equipment is operated with hazardous
voltages that can shock, burn, or cause death. To reduce the
possibility of electrical shock and inadvertent UV exposure,
do not remove any fixed panels. Ensure that all service to the
System, beyond what is described in this manual, is
performed only by qualified Avedro service personnel.

Mosaic Operator’s Manual Rev F| Page
4
ML-00023
WARNING: Power down System and remove the wall plug
before servicing or cleaning (disinfecting) the equipment.
Never pull cords to remove the power cord from the outlet.
Grasp the power cord plug and pull it from the outlet to
disconnect.
The equipment must be positioned so that it is not difficult
to remove the power cord from the outlet.
WARNING: Do not operate the equipment with a damaged
power cord.
WARNING: Position the power cord so that it cannot be
tripped over, walked on, rolled over, crimped, bent, pinched,
or accidentally pulled from the wall outlet.
WARNING: Do not use the instrument near water and be
careful not to spill liquids on any part of it.
WARNING: Do not operate the Mosaic System in the
presence of flammable mixtures or anesthetics.
WARNING: Never look directly into the UV light beam. Never
direct the beam towards a person except for therapeutic
purposes.
WARNING: Ignoring local regulations on use of electro-
optical medical devices may cause malfunction due to
electromagnetic interference.
WARNING: Use of not included accessories results in non-
compliance of the device.
WARNING: System may be interfered with by other
equipment even if that equipment complies with CISPR
Emissions requirements. See Table 5-1.

AVEDRO | 5of 62
ML-00023
WARNING: Use of this equipment adjacent to or stacked
with other equipment should be avoided because it could
result in improper operation. If such use is necessary, this
equipment and the other equipment should be observed to
verify that they are operating normally.
WARNING: Portable RF communications equipment
(including peripherals such as antenna cables and external
antennas) should be used no closer than 30cm (12 inches) to
any part of the Avedro Mosaic System (110-03029), including
cables specified by the manufacturer. Otherwise,
degradation of the performance of this equipment could
result.
WARNING: System shall not be serviced or maintained while
in use with a Patient.
WARNING: MR Unsafe – Keep away from magnetic
resonance imaging equipment.
WARNING: Do not use a damaged or malfunctioning device.
Use of such devices may harm the user and/or patient.
1.7.4 Radiation Safety Warnings
WARNING: Use only laser grade instruments in order to
prevent reflected UV radiation from smooth metallic
surfaces.
WARNING: UV emitted from this product. Avoid eye and skin
exposure to unshielded products. Never direct the beam
towards a person except for therapeutic purposes.

Mosaic Operator’s Manual Rev F| Page
6
ML-00023
Patient Safety
The treatment should take place in a quiet and relaxed atmosphere in
order not to distract the attention of the patient.
•The patient should lie on a table or patient's chair.
•The patient’s head should rest comfortably in a headrest. It is
imperative that the table or patient’s chair and the System not
be moved during the treatment procedure.
CAUTION: The Mosaic System is a medical device. It may be
operated, therefore, only in health care facilities or medical
areas under the supervision of medically trained personnel.
Additional Safety Considerations
•UV irradiance of the Mosaic System is calibrated by the
manufacturer. Any modification of the System's external light
beam by means of optical elements is strictly prohibited.
•Plastic instrumentation such as speculums or eye shields may
be damaged when impacted by the UV beam, possibly resulting
in product degradation. Therefore, only Avedro recommended
accessories or stainless steel surgical instruments should be
used.
•Smooth metallic surfaces can reflect despite the effort to blank
them. Therefore, only laser grade instruments should be used.
FCC Compliance Notice
This equipment has been tested and found to comply with the limits for a
Class B digital device, pursuant to Part 15 of the FCC Rules. These limits
are designed to provide reasonable protection against harmful
interference in a residential environment.
This equipment generates, uses, and can radiate radio frequency energy
and, if not installed and used in accordance with this Manual, may cause
harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation.
If this equipment does cause harmful interference to radio or television
reception, which can be determined by turning the equipment off and on,
the user is encouraged to try to correct the interference by one or more
of the following measures:
•Reorient or relocate the receiving antenna.
•Increase the separation between the equipment and receiver.

AVEDRO | 7of 62
ML-00023
•Connect the equipment into an electrical outlet on a circuit
different from that to which the receiver is connected.
•Consult Avedro Customer Service for help.
Properly shielded and grounded cables and connectors must be used in
order to meet FCC emission limits. Proper cables and connectors are
available from Avedro. Avedro is not responsible for any radio or
television interference caused by unauthorized changes or modifications
to this equipment. Unauthorized changes or modifications could void the
user's authority to operate the equipment.

Mosaic Operator’s Manual Rev F| Page
8
ML-00023
2Introduction
System Overview
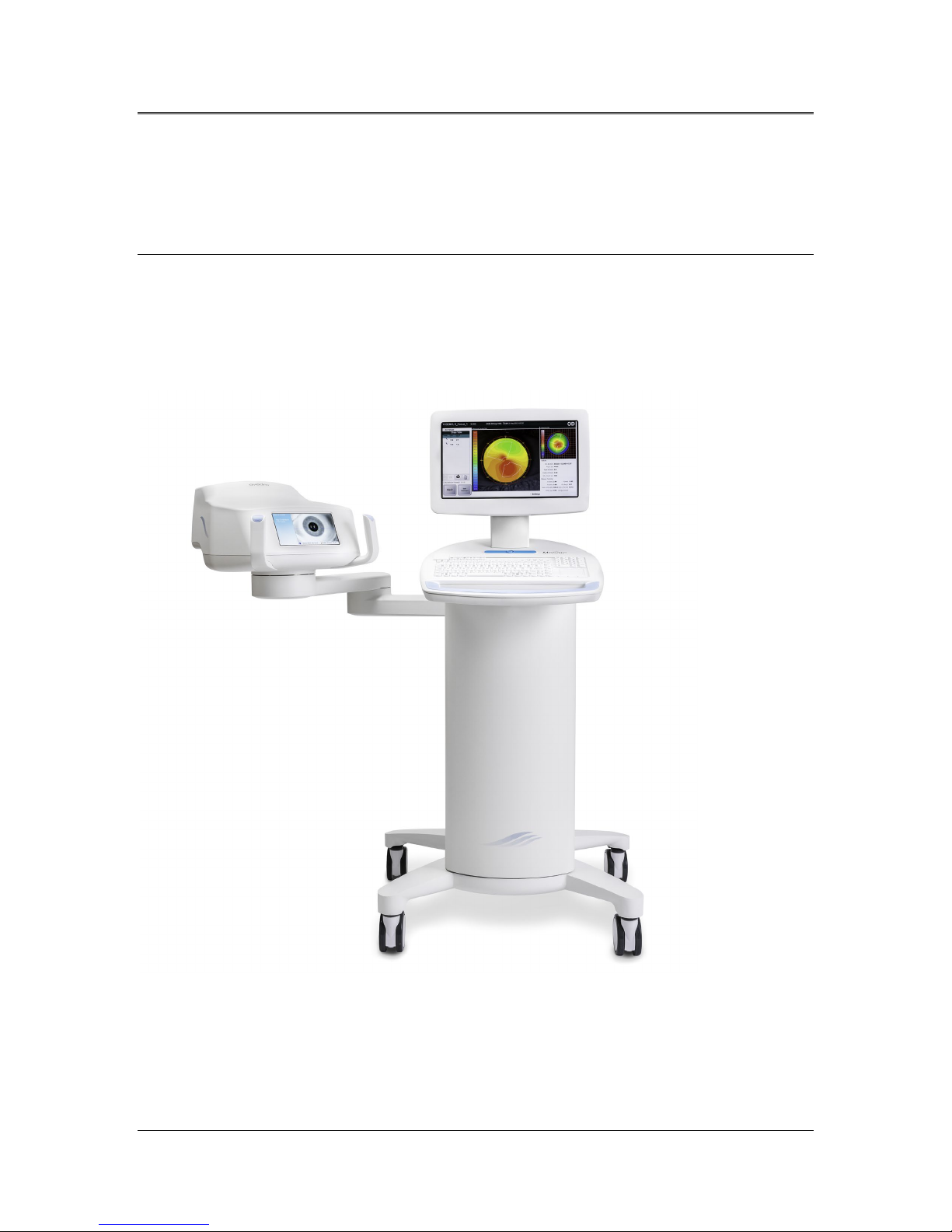
The Mosaic System is an electronic medical device which delivers
ultraviolet light (365 nm wavelength) in a programmable pattern onto the
cornea after a solution of ophthalmic riboflavin has been applied.
Irradiating the riboflavin creates singlet oxygen, which forms
intermolecular bonds in corneal collagen, stiffening the cornea through
cross-linking. UV flux and irradiation time (i.e., fluence) at the cornea are
controlled by an onboard computer system.
The Mosaic is a portable system with an articulating arm to allow
movement of the System for alignment of the UV beam to the patient’s
cornea. The Optics Head houses the UV irradiation mechanism. The height
of the Optics Head can be adjusted through a lift in the body of the
System. The LED is preset by the manufacturer to emit UV radiation at a
wavelength of 365 nm at an intensity of 10 mW/cm2to 100 mW/cm2
(±10%).
The treatment parameters (Riboflavin Induction Period, Total UV Energy,
UV Treatment Patterns, UV Power and UV Pulse Cycle Times) are selected
through the user interface touchscreen.
The Mosaic System is used in conjunction with an ophthalmic riboflavin
solution and a Treatment Activation card.
NOTE: The depictions of the Mosaic System and user interface
screenshots included in this manual are for demonstration purposes
only. Actual product may vary due to product enhancements.

AVEDRO | 9of 62
ML-00023
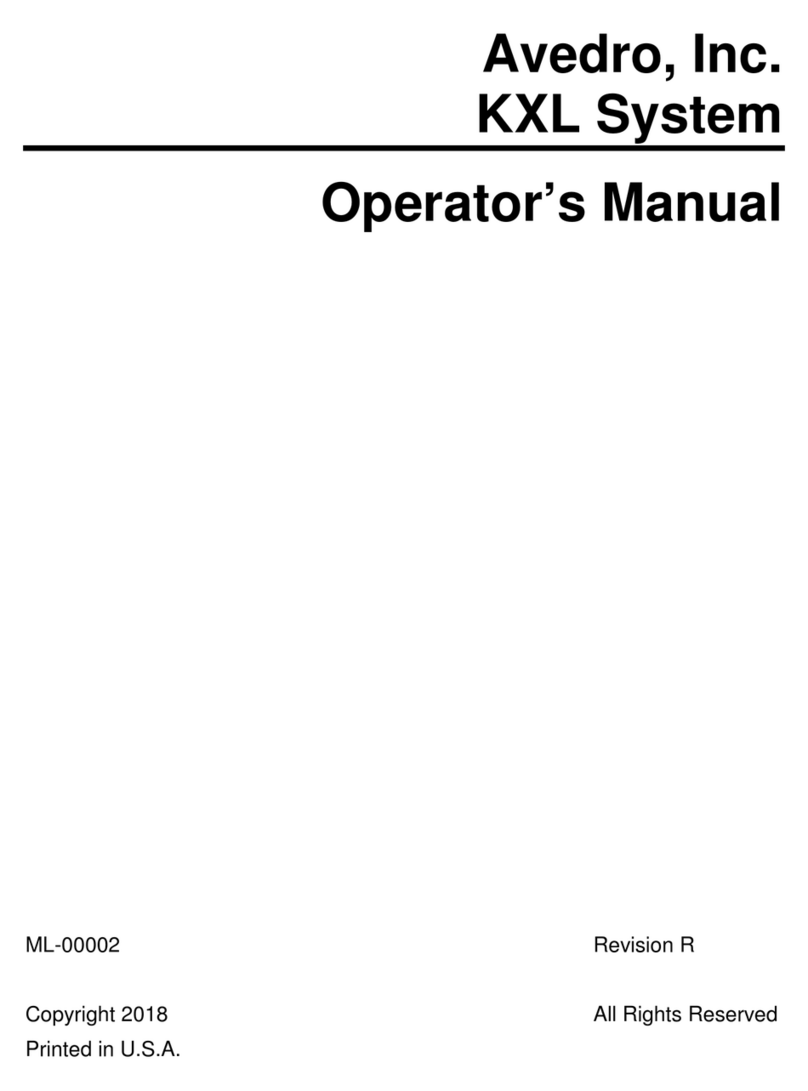
2.1.1 Major Components
The major components of the Mosaic System include the following:
•Articulating arm with optics head with UV source and heads up
display
•Mosaic console with user interface
•Mosaic Accelerated Crosslinking Treatment Kit (disposable
supplied separately)
•USB flash drive port
•System Part Number: 110-03092
Figure 2-1. Overview Illustration of Mosaic System

Mosaic Operator’s Manual Rev F| Page
10
ML-00023
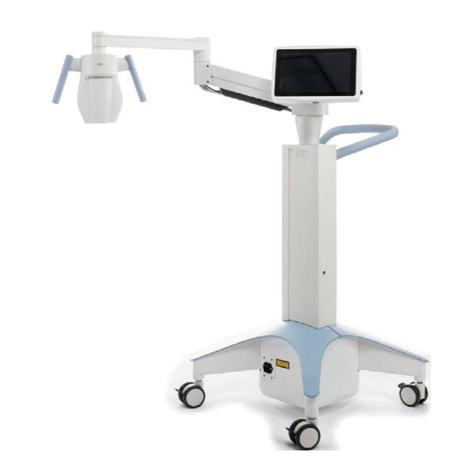
Figure 2-2. System Overview with Callouts
2.1.2 Disposables
The following disposables (supplied separately) are required for
performing treatments with the Mosaic System:
•Riboflavin ophthalmic solutions
•Treatment Activation card
•Boost Goggles (optional)
B
C
D
E
F
G
H
A
AMain Console with USB flash drive
port and Treatment Card slot
BSystem Keyboard
CSystem Body with lift system
DArticulating Arm
EHeads Up Display
FOptical Head
GStand by Switch
H Wheels

AVEDRO | 11 of 62
ML-00023
2.1.3 Mosaic System Labels
Figure 2-3. Mosaic Label
Figure 2-4. UV Safety Label
Figure 2-5. Laser Classification Label
Figure 2-6. Do Not Sit/Step On Label

Mosaic Operator’s Manual Rev F| Page
12
ML-00023
2.1.4 Frequently Used Functions
The table below identifies the System functions that are most
frequently used and where to find these functions described in this
Manual.
Function
Mode
Manual
Section
Turn System On Press Stand By switch under the keyboard in
the front of the Mosaic console
4.3
Treatment
Design
Select “Design OD”/ “Design OS”. This initiates
a new treatment design.
5.2
Scan Treatment
Activation Card
Insert Treatment Activation card into slot on
the left side of main console
5.3
Begin UV
Treatment
Select “Begin UV Treatment”. This becomes
available at the end of the patient preparation
step.
5.10
Pause Select “Pause” to interrupt treatment at any
time.
5.10
Go on Stand By Select “Power Off” on main console display 5.13
AVEDRO | 13 of 62
ML-00023
3System Operation
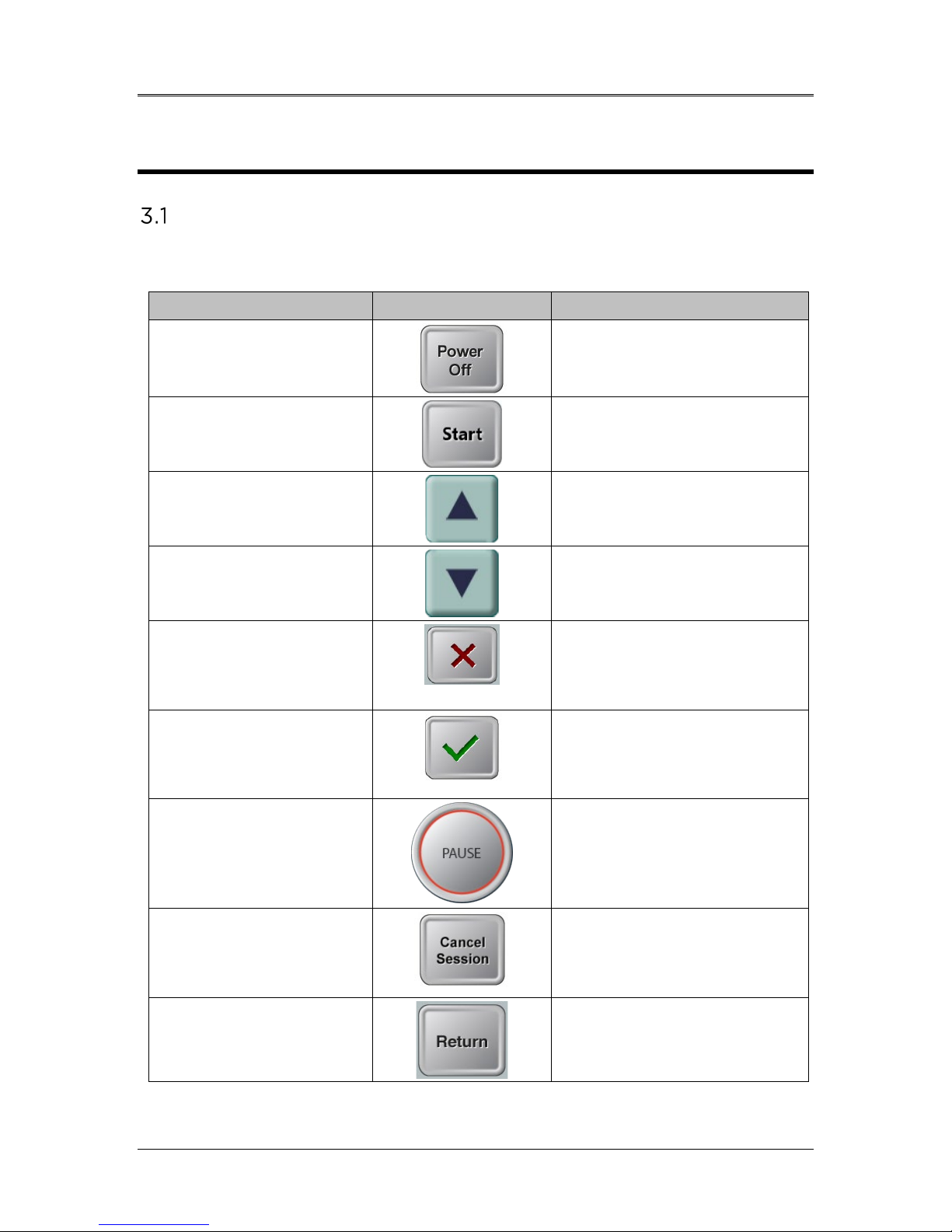
Touchpad/Keyboard Use
The table below identifies and describes important touchpad keys and
icons unique to Mosaic System operation.
Touchpad Key
Icon
Description/Function
Power Off button
(Initial screen)
Turns OFF electric power to
the console and goes on
Stand By Mode.
Start button (initial
screen)
Directs the System from the
Startup Screen to Patient
Central.
UP arrow
(various Clinical
Protocol screens)
Increases the value of the
current field.
DOWN arrow
(various Clinical
Protocol screens)
Decreases the value of the
current field.
X button
(various Device Settings
screens)
Cancels all the entries on a
particular screen and
returns to the previous
screen.
Checkmark button
(various Clinical
Protocol screens and
Device Settings screen)
Directs the System to
accept the current screen
entries and to proceed to
the next step.
Pause button
(Treatment screen)
Halts the delivery of UV
light until the physician
decides to realign the eye,
resume, or cancel the
treatment session.
Cancel Session button
(various Clinical
Protocol screens)
Cancels a treatment session
for a particular patient. A
prompt is then displayed to
confirm your decision.
Return button
(various Device Settings
screen)
Returns to the Device
Settings menu.

Mosaic Operator’s Manual Rev F| Page
14
ML-00023
CAUTION: Only qualified and experienced personnel shall
operate the Mosaic System.
UV Energy (Dose)
•The UV Energy (Dose) is the product of the UV Power
(Intensity) and the UV Irradiation Time. The UV Energy, UV
Power and pulsing times are adjustable and the calculated total
treatment time is displayed.
•The System tracks UV Energy, UV Power, UV Irradiation Time
and Total Treatment Time during the treatment.
•Treatment Parameters:
-Induction Period: 1 second – 30 minutes
-UV Energy: 0 – 15 J/cm2
-UV Power: 10 – 100 mW/cm2(± 10%)
•Please reference Riboflavin Instructions for Use (IFU) for
formulation information.
Table of contents
Other Avedro Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Buffalo filter
Buffalo filter PlumeSafe 1202 Operator's manual
Johnson & Johnson
Johnson & Johnson ETHICON LAPRA-TY Suture Applier Optimized Device Performance Guide
Siare
Siare Siaretron 4000 Service manual

Beurer
Beurer HK 44 Instructions for use

Nipro
Nipro SURDIAL Service manual

Aeonmed
Aeonmed Aeon7400A Service manual