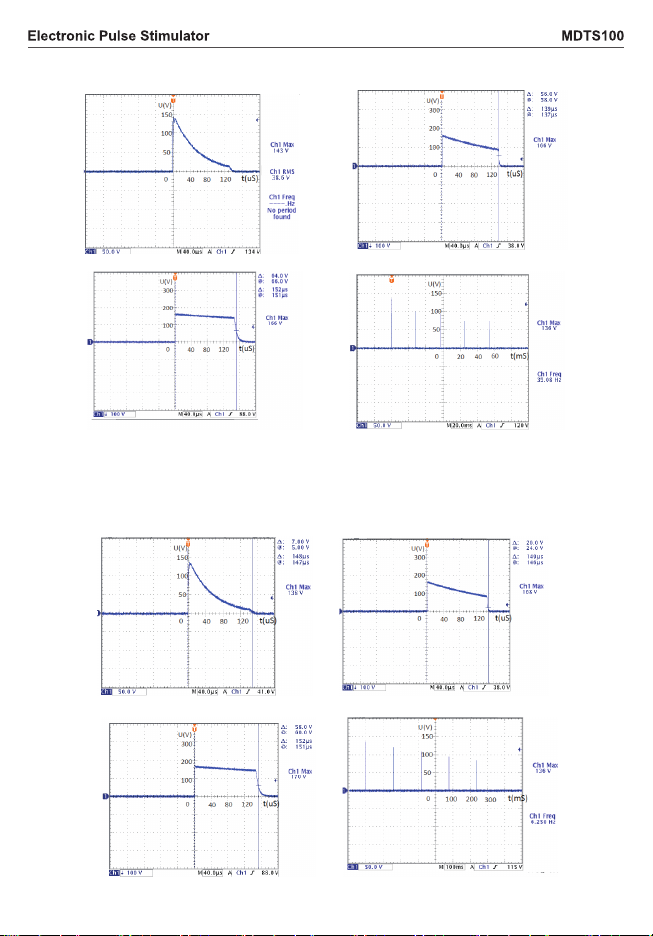
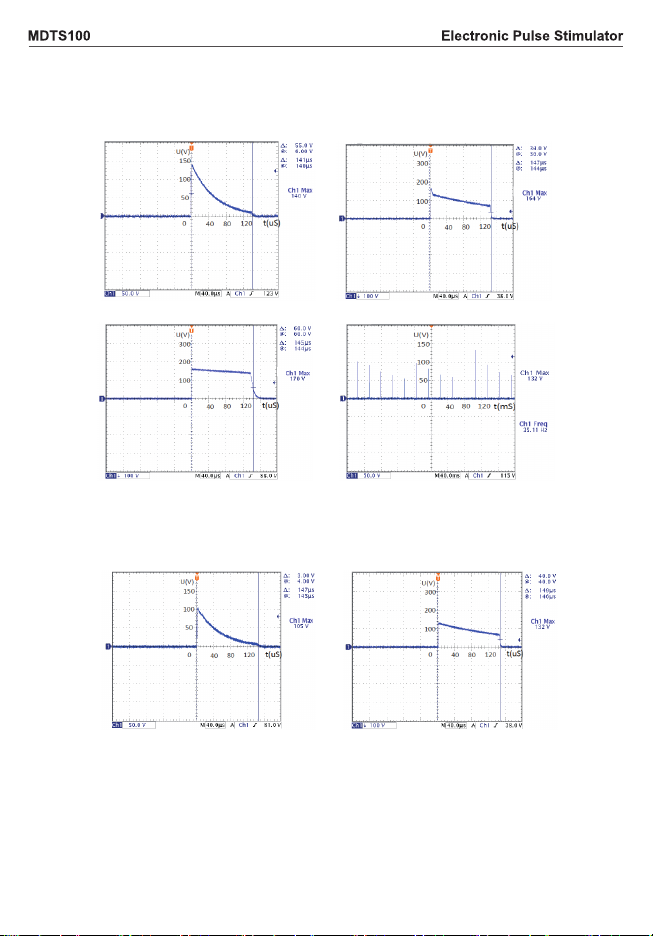
2
• 10 Levels of intensity
• 8 Positions (LEG, SOLE, FOOT, BACK, SHOULDER, HAND, JOINT and WAIST)
• 20 Minutes Timer
7 Safety Warnings
DANGER
• Do not use this device if you have an implanted debrillator or implanted metallic devices,
such as a pacemaker. Such use could cause electrical shock, burns, electrical interference
or death.
• Don't use the device if you use a high frequency surgical ME EQUIPMENT simultaneously.
Such use may result in burns and possible damage to the device.
• Operation in close proximity (e.g. 1m) to a shortwave or microwave therapy ME
EQUIPMENT may produce instability in the device output.
• The application of electrodes near the thorax may increase the risk of cardiac brillation.
WARNING
• Use of accessories and cables other than those specied or provided by the manufacturer
of this equipment could result in increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and result in improper operation.
• Portable RF communications equipment (including peripherals such as antenna cables
and external antennas) should be used no closer than 30 cm (12 inches) to any part of the
ME EQUIPMENT, including cables specied by the manufacturer. Otherwise, degradation
of the performance of this equipment could result.
• Use of this equipment adjacent to or stacked with other equipment should be avoided
because it could result in improper operation. If such use is necessary, this equipment and
the other equipment should be observed to verify that they are operating normally.
NOTICE
• Do not use this device while driving or sleeping.
• Do not use this device in high humidity areas such as a bathroom.
• Keep the device away from wet, high temperature and direct-sunlight place.
• Keep this device out of reach of children.
• Stop using this device and consult your physician immediately if you feel pain, discomfort,
dizziness or nausea.
• Do not attempt to move the electrode pads while the device is ON or operating.
• Any electrodes that have current densities exceeding 2mA/cm2 may require special
attention of the operator.
• Do not use the device around the heart, on the head, mouth, genitals or blemished skin
areas, on the front of the neck, or from electrodes placed on the chest and the upper back
or crossing over the heart.
• When the device delivers an output of more than 10mA or 10V into a load resistance of
1000Ω, the device will have an indication that the A&B channels font ashes on the display
screen.
Do not apply stimulation of this device in the following conditions:
1. Across the chest-the introduction of electrical current into the chest may cause rhythm
disturbances to the heart, which could be lethal.
2. Over painful areas. Consult your physician before using this device if you have painful
areas.
3. Over open wounds, rashes, or swollen, red, infected, or inamed areas or skin eruptions
(e.g. phlebitis, thrombophlebitis, varicose veins). Apply stimulation only to normal, intact,