Clinomic Mona Terminal User manual

Mona Terminal
Operating instructions
Hardware version: 1.1
English Read the instructions prior to performing any task!

Gebrauchsanweisung, 6, en_GB
© Clinomic GmbH 2021
Clinomic GmbH
Bachstr. 22
52066 Aachen
Nordrhein-Westfalen
GERMANY
Telephone: +49 241 89438737
Email: [email protected]
Internet: www.clinomic.ai
11.08.2021 Mona Terminal2
This instruction manual enables safe and efficient handling of the
Mona terminal. This instruction manual is a component of the ter-
minal and must be kept in the immediate vicinity of the terminal
where it is accessible to personnel at all times.
Personnel must have carefully read and understood the instruction
manual before beginning any work with the product. Compliance
with the safety information and instructions provided in this instruc-
tion manual is an essential prerequisite for safe use of the product.
In addition, the local occupational health and safety regulations
and the general safety regulations for the area in which the ter-
minal is used must be observed.
Illustrations in this instruction manual are intended for basic under-
standing and may deviate from the actual design.
The terminal only functions with the corresponding MonaOS soft-
ware. Operation of the terminal is based on the software functions.
The relevant instructions can be found in the software manual
Ä
“Other applicable documents” on page 3.
Clinomic customers will be informed if and when future revisions of
this instruction manual are made available.
The content of this instruction manual is protected by copyright.
Use of this content is permitted within the context of using the ter-
minal. Any other use is prohibited without the written approval of
Clinomic GmbH.
The documents listed below apply in addition to this instruction
manual.
Document Note
MonaOS software manual – AI
assistance software for inten-
sive care
Note the software version level
of MonaOS
Manual for remote users of
MonaOS – telemedicine web
interface
Note the software version level
of MonaOS
Spring arm24 SKYDOQ
instruction manual The spring arm24 Standard ver-
sion (STD) is used with an
appropriate adapter.
Data sheet for Intel
9260.NGWG Wireless Wi-Fi Bluetooth
adapter
Data sheet for TWN4 MULTI-
TECH 3 LF RFID chip reader
Data sheet for Quectel
RM500Q-GL 5G module
About this instruction manual
Copyright
Other applicable documents
11.08.2021 Mona Terminal 3

In the interests of monitoring our products, we are interested in
information and experience relating to use of the terminal and this
instruction manual. We would therefore be very grateful for rele-
vant feedback. If you are unsure about any of the information given
in this instruction manual, please feel free to contact us.
If you experience any serious incidents involving the product,
please contact Clinomic GmbH without delay, as well as the
responsible authorities in the EU Member State, where relevant.
Product monitoring
11.08.2021 Mona Terminal4

Table of contents
1 Structure and function....................................................... 7
1.1 Functional description................................................... 7
1.2 Functional elements and connections.......................... 8
1.3 Scope of delivery........................................................ 10
2 Safety................................................................................. 11
2.1 Symbols in this instruction manual.............................. 11
2.2 Intended purpose........................................................ 12
2.3 Residual risks............................................................. 14
2.4 Property damage........................................................ 17
2.5 Specialist qualifications............................................... 18
2.6 Necessary equipment and resources......................... 19
2.7 Environmental protection............................................ 20
3 Transport and storage...................................................... 21
4 Installation and connection............................................. 22
4.1 Preparing and configuring the terminal....................... 22
4.2 Installing the terminal.................................................. 22
5 Operation........................................................................... 25
5.1 Switching the terminal on and off................................ 25
5.2 Operating the terminal................................................ 25
5.3 Cleaning and disinfecting the terminal........................ 27
6 Maintenance...................................................................... 28
7 Malfunctions...................................................................... 29
7.1 List of possible malfunctions....................................... 29
7.2 Rectifying system faults.............................................. 30
8 Technical specifications................................................... 32
8.1 Information on the type plate...................................... 32
8.2 Device classification................................................... 33
8.3 Module specifications................................................. 33
8.4 Accessories................................................................ 34
8.5 Dimensions and weight............................................... 35
8.6 Performance data....................................................... 35
8.7 External connection.................................................... 35
8.8 Requirements for the ambient conditions................... 36
8.9 EMC and requirements from the electrical stan-
dards........................................................................... 36
8.9.1 Electromagnetic compatibility (EMC) require-
ments....................................................................... 36
8.9.2 Electromagnetic immunity........................................ 37
8.9.3 Recommended safety distances.............................. 39
9 Disposal............................................................................. 42
10 Index................................................................................... 43
Appendix............................................................................ 46
Table of contents
11.08.2021 Mona Terminal 5
1 Structure and function
1.1 Functional description
The Mona terminal is an assistance system for intensive care units
that helps nursing staff and doctors provide patients with the best
possible care. It consists of the terminal and the MonaOS software
operated on the terminal.
The system can be used for all patients being treated in an inten-
sive care unit. It provides various functions – depending on which
MonaOS software version is used – to aid medical documentation
in intensive care units.
The terminal can be attached to a wall or ceiling bracket in the rele-
vant care environments (accident and emergency units, intensive
care units, operating theatres, anaesthetic recovery rooms etc.).
The terminal is operated using a touch screen.
Depending on which version of MonaOS is used,
other interactive features may be available.
The system is connected to the hospital’s digital infrastructure
(hospital information and auxiliary systems) using a wireless LAN
or LAN connection.
In addition, the terminal has the following wireless connections that
are used for the enhanced functions and for the access authorisa-
tion process:
Technology Use Additional information
RFID User authentication
with RFID tags
Ä
“TWN4 MULTITECH 3
LF (RFID chip reader)”
on page 34
4G Data transmission
for video calls
Ä
“Quectel RM500Q-GL
(5G module)”
on page 34
Bluetooth Communication
with other devices
Ä
“Intel 9260.NGWG
(wireless Wi-Fi Bluetooth
adapter)” on page 33
Area of application
Point of use and interaction
Interfaces and system connection
Structure and function
Functional description
11.08.2021 Mona Terminal 7
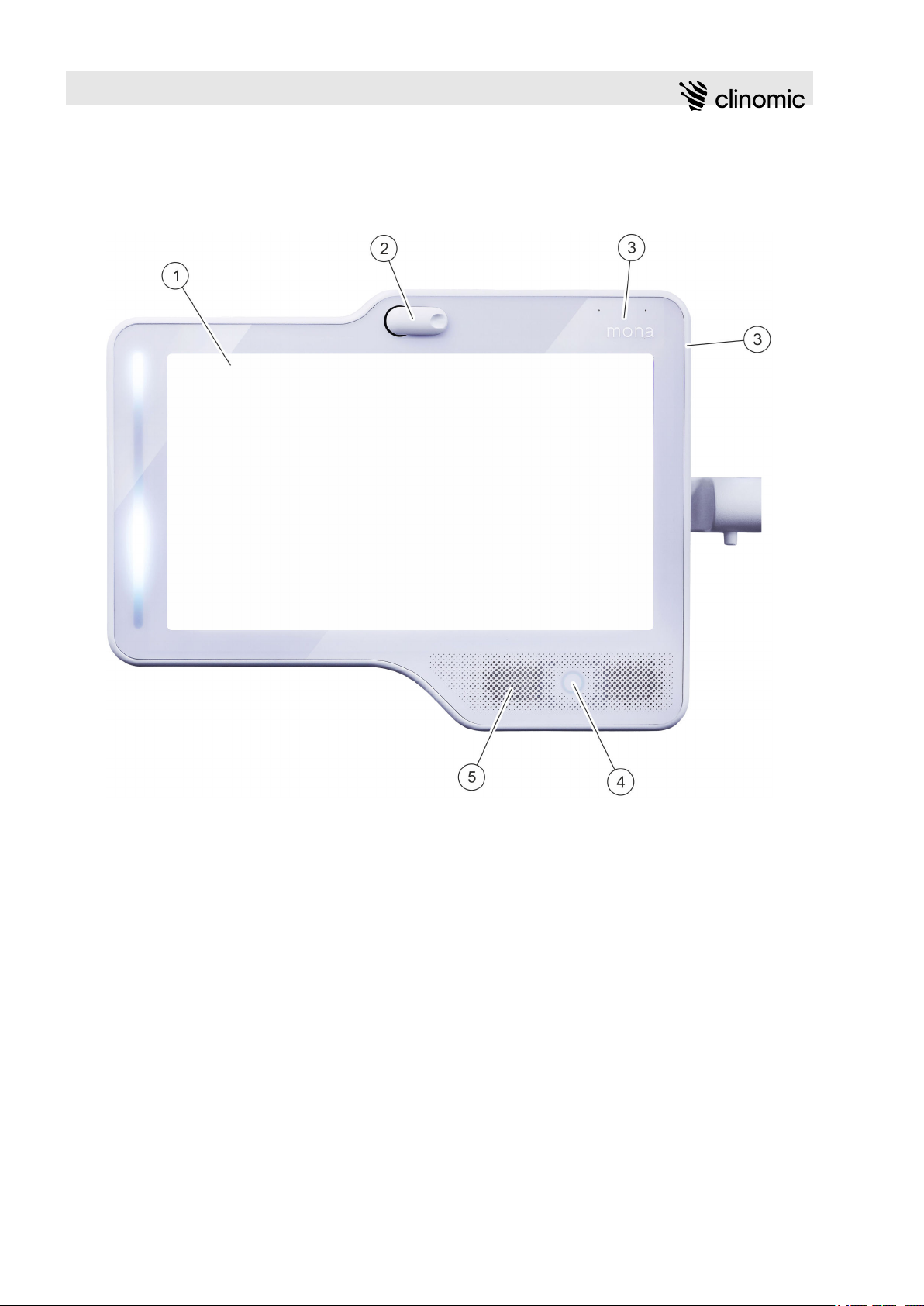
1.2 Functional elements and connections
Fig. 1: Front view
1 Touch screen
2 Camera
3 Front microphones (2x)
4 RFID recognition
5 Loudspeaker
Front
Structure and function
Functional elements and connections
11.08.2021 Mona Terminal8

Fig. 2: Rear view
1 Rear microphones (2x)
2 Ethernet connection (network connection)
3 USB-A port
4 Power connection (on the underside)
5 On/off switch
6 Fastening screw thread for VESA adapter (4x)
The software is operated by tapping the touch screen with your
finger.
The terminal is switched on and off at the on/off switch.
The terminal features ambient lighting. The lighting
can light up when you interact with the terminal, in
order to provide feedback to the user.
See the software manual.
Back
Touch screen (Fig. 1/1)
On/off switch (Fig. 1/5)
Lighting
Structure and function
Functional elements and connections
11.08.2021 Mona Terminal 9

1.3 Scope of delivery
nMona terminal
nInstruction manual
Structure and function
Scope of delivery
11.08.2021 Mona Terminal10
2 Safety
2.1 Symbols in this instruction manual
Safety information is indicated in this instruction manual by sym-
bols. The safety information is introduced by signal words that indi-
cate the magnitude of the hazard.
In order to avoid accidents, injuries and property damage and to
ensure the greatest possible patient safety, always follow the
safety instructions and proceed with caution.
DANGER!
This combination of symbol and signal word indi-
cates an imminently hazardous situation that will
result in death or severe injuries if it is not avoided.
WARNING!
This combination of symbol and signal word indi-
cates a potentially hazardous situation that may
result in death or severe injuries if it is not avoided.
CAUTION!
This combination of symbol and signal word indi-
cates a potentially hazardous situation that may
result in minor or slight injuries if it is not avoided.
NOTICE!
This combination of symbol and signal word indi-
cates a potentially hazardous situation that may
result in property damage and/or environmental
damage if it is not avoided.
This symbol highlights useful tips and recommen-
dations, as well as information that helps ensure
efficient and trouble-free use of the device.
The following signs are used in this instruction manual to highlight
instructions, results, lists, cross-references and other elements:
Safety information
Tips and recommendations
Signs in this document
Safety
Symbols in this instruction manual
11.08.2021 Mona Terminal 11

Sign Explanation
Step-by-step instructions
ðResults of actions
References to sections of this instruction
manual
Lists with no specified order
2.2 Intended purpose
The Mona terminal enables efficient interaction between medical
specialists within an intensive care unit by making appropriate
hardware and software components available on a single device,
in order to support medical specialists in providing treatment.
The Mona terminal is a device that provides the hardware and
operating system infrastructure for the MonaOS software. The
Mona terminal is designed to be used in combination with the
MonaOS software.
The Mona terminal supports the MonaOS software in the fol-
lowing areas:
nMedical documentation by means of voice recognition, hard-
ware and software components, and modules
nDisplaying information that is created and controlled by
MonaOS on special monitors and screens
nProviding a user interface runtime environment for operating
the MonaOS software
nProviding the computing infrastructure for the MonaOS soft-
ware
The precise scope of functions is defined by the version of the
MonaOS software that is used.
The intended purpose includes compliance with all the information
in this instruction manual.
Any use beyond or other than the intended purpose is considered
misuse.
Safety
Intended purpose
11.08.2021 Mona Terminal12

WARNING!
Danger in the event of misuse!
Misuse of the terminal can result in hazardous sit-
uations.
– Never use the terminal for mobile emergency
care (e.g. ambulance).
– Never use the terminal in residential care.
– Never allow unauthorised persons to access the
terminal.
– Never open the terminal’s housing.
– Do not stack the terminal with other devices.
– Never lay the power supply cables for other
devices across the terminal or wind them
around the terminal’s mounting elements.
The terminal can be used in combination with the MonaOS soft-
ware for all patients who are treated in an intensive care unit.
There are no contraindications or exceptions to the use of the ter-
minal in combination with the MonaOS software.
There are no reciprocal effects in the use of the terminal in combi-
nation with the MonaOS software.
A further intended purpose is regular cleaning of the terminal by
means of wipe disinfection once per shift.
We differentiate between the following groups of persons who are
authorised to operate the terminal as specialist personnel:
Medical specialists (principal
operators) Doctors and nursing staff in
intensive care units
Service staff (secondary opera-
tors) Specialists responsible for
installation, updates and config-
uration
Specialists responsible for dis-
infecting medical devices
Not all groups of persons are relevant to this
instruction manual.
Indications
Contraindications
Reciprocal effects
Further intended purpose
Personnel characteristics
Safety
Intended purpose
11.08.2021 Mona Terminal 13

The patients are critically ill patients who are being treated in an
acute care unit, such as an accident and emergency unit, intensive
care unit, operating theatre, anaesthetic recovery room etc.
Use of the terminal in combination with the MonaOS software is
not restricted to specific illnesses, comorbidities or demographic
characteristics.
2.3 Residual risks
DANGER!
Risk of fatal electric shock!
Contact with live parts poses an immediate danger
of fatal electric shock. Damage to the device or the
power supply cable can be life-threatening.
– Make sure that the building’s mains connection
has a 4 kV isolation barrier (network isolator).
– Only connect the device to a mains supply with
a protective earth conductor.
– To isolate the device from the mains supply,
unplug the power supply cable.
– Keep moisture away from the device and the
power supply cable. Failure to do so can result
in a short circuit.
– If the device is damaged, switch it off immedi-
ately, put it out of use and arrange for repairs.
– If the power supply cable is damaged, switch off
the device immediately and replace the power
supply cable.
– Have defective devices repaired by Clinomic
customer service only.
Patient characteristics
Electric current
Safety
Residual risks
11.08.2021 Mona Terminal14
WARNING!
Risk of infection in the event of insufficient
hygiene and disinfection!
There is a risk of infection in the event of contact
with parts of the device that have not been cleaned
and disinfected.
– Clean and disinfect the device at least once per
shift
Ä
Chapter 5.3 “Cleaning and disinfecting
the terminal” on page 27. If local circum-
stances require more frequent cleaning and dis-
infection, clean and disinfect the device more
often accordingly.
– The device may only be cleaned with the
described cleaning materials as described in
Ä
Chapter 5.3 “Cleaning and disinfecting the
terminal” on page 27.
– Note the information on the type of disinfection
and the disinfectants to use.
Risk of infection
Safety
Residual risks
11.08.2021 Mona Terminal 15
WARNING!
Danger due to failure to comply with the elec-
tromagnetic compatibility requirements!
Medical electrical equipment is subject to specific
electromagnetic compatibility (EMC) requirements.
Failure to comply with the safety requirements can
result in malfunctioning of the device and it poses
a risk of adverse effects on other equipment, which
can in turn result in damage, malfunctions or even
total failure, with corresponding dangers to
patients.
Make sure that the device is installed and operated
in accordance with the following specifications:
– Only use connecting cables recommended by
the manufacturer
Ä
Chapter 8.4 “Accessories”
on page 34.
– Do not use any accessories other than those
described and sold by the manufacturer. Spare
parts that have not been produced or approved
by the manufacturer may increase electromag-
netic interference emissions or impair the devi-
ce’s electromagnetic immunity.
– Wearable HF communication devices (including
peripheral devices such as antenna cables and
external antennas) should not come closer than
30 cm (12 inches) to any component of the
Mona terminal, including the cables and lines as
specified in these instructions
Ä
Chapter 8.9.3
“Recommended safety distances” on page 39.
Otherwise the performance of the device can be
affected.
– The use of this device with neighbouring
devices or stacked with other devices should be
prevented, as it could result in incorrect opera-
tion. If such an application is required, this
device and the other devices should be moni-
tored to ensure that the devices behave nor-
mally.
– The operator and the patient must not come into
physical contact with one another while the
device is being operated.
Electromagnetic compatibility
Safety
Residual risks
11.08.2021 Mona Terminal16
WARNING!
Risk of injury due to the use of unsuitable
spare parts or incorrect accessories!
Using unsuitable or faulty spare parts or accesso-
ries can result in dangers to personnel, as well as
damage, malfunctions or complete failure.
– Only use genuine spare parts and accessories
from Clinomic or approved by Clinomic
Ä
Chapter 8.4 “Accessories” on page 34.
– Do not make any technical modifications.
– If in doubt, always contact Clinomic customer
service.
CAUTION!
Risk of injury from falling device!
If the device is not properly mounted, it may fall
down and cause injuries.
– Only mount the device on a spring arm
designed for that purpose, using the corre-
sponding adapter plate
Ä
Chapter 8.4 “Acces-
sories” on page 34.
– Make sure that the device is fastened properly
during the course of installation.
2.4 Property damage
NOTICE!
Overloading of the USB ports if unsuitable
peripheral equipment is connected!
If equipment with a high current consumption is
attached to the USB port, it may cause overloading
and damage to the USB port.
– Do not operate any USB devices on the USB
port. The USB port is intended solely for service
tasks performed by Clinomic customer service.
Unsuitable spare parts and acces-
sories
Falling
USB port
Safety
Property damage
11.08.2021 Mona Terminal 17
NOTICE!
Damage to the microphones if handled incor-
rectly!
Improper handling may result in electrostatic dis-
charge, which can damage the built-in micro-
phones.
– Do not touch the microphone openings on the
terminal.
Electrostatic discharge cannot impair any of the
fundamental device functions.
NOTICE!
Damage to the terminal if liquids penetrate it!
Liquids can enter the terminal through slots and
openings in the housing and cause damage.
– Do not store any liquids in the immediate vicinity
of the terminal that could spill into the terminal if
knocked over.
– When disinfecting the terminal, use only surface
disinfection, not spray disinfection.
– When disinfecting the terminal, make sure that
liquid disinfectant does not leak into the terminal
through slots and openings.
2.5 Specialist qualifications
WARNING!
Danger if personnel are insufficiently qualified!
If unqualified personnel carry out work or make
adjustments on the terminal, there is a risk of injury
and property damage.
– All work and adjustment on and to the terminal
must be carried out by qualified specialists only.
– Keep unqualified personnel away from the ter-
minal.
Electrostatic discharge
Liquids
Safety
Specialist qualifications
11.08.2021 Mona Terminal18

This instruction manual specifies the following personnel qualifica-
tions for different areas of activity:
Medical specialists (principal operators)
The medical specialists (principal operators) are doctors and
nursing staff in intensive care units. Thanks to their professional
education, medical specialists are able to carry out the duties
entrusted to them.
Service personnel (secondary operators)
Service personnel are Clinomic employees or personnel trained by
Clinomic who are responsible for IT administration (installation,
configuration, updates) (hospital IT department). Thanks to their
professional education and specific training, service personnel are
able to carry out the duties entrusted to them.
The group of secondary operators also includes specialists who
are responsible for disinfecting medical devices.
Only persons who can be expected to perform their tasks reliably
are permitted as personnel. Persons whose reactions are impaired,
e.g. due to drugs, alcohol or medication, are not permitted.
When selecting personnel, observe the locally applicable age-spe-
cific and professional regulations.
2.6 Necessary equipment and resources
The following equipment is needed for certain activities on the
device:
Hexagon socket wrench SW4
Hexagon socket wrench with wrench size 4
Torque wrench, tightening torque at least 10 Nm
Torque wrench with a tightening torque of at least 10 Nm
The following resources are needed for certain activities on the
device:
Disposable disinfectant wipes
Disposable disinfectant wipes for wiping surfaces that require med-
ical disinfection.
Fastening screws: 4 pieces; M5x20; A2-70 DIN 912 (included
in scope of delivery of spring arm)
Fastening material for installation of the terminal on the spring arm
Surface disinfectant
Approved disinfectant for medically disinfecting surfaces.
Washers: 4 pieces; washer ISO 7089-5-200 HV-A2 (included in
scope of delivery of spring arm)
Fastening material for installation of the terminal on the spring arm
Qualifications
Safety
Necessary equipment and resources
11.08.2021 Mona Terminal 19

If special equipment or resources are necessary
for specific activities, these are specified at the
start of the respective chapter.
2.7 Environmental protection
ENVIRONMENT!
Danger to the environment due to improper
handling of environmentally hazardous sub-
stances!
If environmentally hazardous substances are han-
dled incorrectly, in particular if they are disposed of
incorrectly, there is a risk of severe damage to the
environment.
– Always comply with the information specified
below on handling and disposing of environ-
mentally hazardous substances.
– If environmentally hazardous substances are
accidentally released into the environment, take
suitable measures immediately. If in doubt,
inform the local authority of the damage and
enquire about suitable measures.
Electronic components may contain environmentally hazardous or
recyclable substances or modules. Collect electronic components
separately and have them recycled or disposed of by authorised
disposal specialists only.
Packaging materials are valuable raw materials and can in many
cases be reused or usefully treated and recycled. If you plan to
transport the terminal or place it in storage, it is useful to retain the
original packaging.
nRecycle or dispose of unneeded packaging materials in an envi-
ronmentally sound manner.
nObserve the locally applicable disposal regulations. If in doubt,
arrange for a specialist company to dispose of the materials.
Electronic components
Packaging materials
Safety
Environmental protection
11.08.2021 Mona Terminal20
Table of contents
Popular Medical Equipment manuals by other brands

Dräger
Dräger BiliLux User quick reference guide

Inogen
Inogen at home user manual

Cardiac Science
Cardiac Science POWER2HEART AED G3 Automatic 9300A Operation and service manual

Nanosonics
Nanosonics trophon AcuTrace Series Instructions for use

Abbott
Abbott Heartmate 3 quick start guide
Bistos
Bistos BT-220 Operation manual