Medset PADSY TELESMART-H/P User manual
I N S T R U C T I O N S F O R U S E
English
TELESMART-H/P
Holter-ECG-Recording System
Version D
PADSYHolter
We have sound ideas.
EN
EN

General nformation
Note:
This user's manual contains essential safety information, in addition to information
on the operation, care and maintenance of the PADSY-Holter software. The safety
information must be read and fully understood before installation and start up. Only
in this manner will the safety of patients and staff be guaranteed. This user's manual
must always be kept with the equipment and accessible to all users.
All rights, particularly translation rights, to the text and illustrations are reserved.
All manner of reproduction is permitted only with the approval of Medset
Medizintechnik GmbH. All and any reprinting, including extracts, and all and any
reproduction of the illustrations, including in a modified state, are prohibited.
Due to continual development, equipment specifications and graphics are subject to
change without notice.
TELESMART-H/P - Holter-ECG-Recording System - version D
General nformation
GBA 17.10.002-05
nstructions for use TELESMART-H/P Holter-ECG-Recording System version D
Version 5
Revised 2013-07
GBA-TS_HP-D-EN.odt
© Medset Medizintechnik GmbH
Medset, PADSY, CARD OL GHT, SCANL GHT, Spirosound, Ergotop are registered trade
marks of Medset Medizintechnik GmbH.
All other trade marks are the property of their respective owners.
Medset Medizintechnik GmbH
Curslacker Neuer Deich 66
D-21029 Hamburg
Germany
Telefon: +49(0)40 / 72 58 22-0
Telefax: +49(0)40 / 72 58 22-11
http://www.medset.com
email://[email protected]m
General nformation
The PADSY-Holter Holter-ECG Recording system bears the CE mark:
According to directive 93/42/EEC of the Council with respect to medical products,
and complies with the fundamental requirements of Appendix of this Directive.
The CE mark includes only those parts listed in the section "Accessories" and in the
CE Declaration of Conformity.
!Sa ety notes, non-compliance with which represents a danger to operators,
patients or the equipment, are visually emphasized by the adjacent graphic
symbol.
Follow instructions for use!
Caution, consult accompanying documentation. Safety precautions need to be
observed that are not stated on the product label!
Follow instructions for use!
Application part of type BF.
Name and address of the product manufacturer
Date of product manufacture, with four numbers to designate the year and,
possibly, two numbers to designate the month.
Denotes the range of humidity, which the medical device can be safely exposed to.
Denotes the range of athmospheric pressure, which the medical device can be
safely exposed to.
TELESMART-H/P - Holter-ECG-Recording System - version D
General nformation
Products marked with this symbol must not be treated as normal household
waste.
The number printed next to this symbol corresponds to the product‘s serial
number.

Seite 6
Contents
1 Application and Function.....................................................................................8
1.1 General..................................................................................................8
1.1.1 Variants.......................................................................................8
1.2 Safety....................................................................................................9
2 Controls and Components...................................................................................12
2.1 Recorder .............................................................................................. 12
2.2 Marker and menu keys.............................................................................12
2.3 Optical and acoustic signals.....................................................................13
3 Equipment startup............................................................................................14
3.1 Special hints for setting up the device with MacOS X ..................................14
4 Operation........................................................................................................ 15
4.1 ECG recording setup................................................................................15
4.1.1 Recorder setup............................................................................15
4.1.2 Skin preparation..........................................................................15
4.1.3 Electrode selection......................................................................16
4.1.4 Electrode application...................................................................17
4.2 Electrode arrangement for patients with pacemakers (TELESMART-H)..............20
4.2.1 Patients with unipolar pacemakers.................................................20
4.2.2 Patients with bipolar pacemakers...................................................21
4.2.3 Patients with mixed pacemaker operation........................................21
4.2.4 Lead scheme using patient cable 3 for pacemaker patients.................21
4.3 Starting an ECG recording........................................................................22
4.3.1 Recorder direct start procedure .....................................................22
4.3.2 Starting the recorder with PADSY...................................................23
4.3.3 External start..............................................................................25
4.4 Patient instruction.................................................................................25
4.5 Ending the ECG recording........................................................................26
4.6 Downloading the ECG recording................................................................26
4.7 Using Accessories...................................................................................27
4.7.1 Disposable/Rechargeable Batteries.................................................27
4.7.2 Battery compartment...................................................................27
4.7.3 Memory card...............................................................................27
4.7.4 Recorder bag...............................................................................28
5 Cleaning and Service.........................................................................................29
5.1 Cleaning and disinfection........................................................................29
5.1.1 TELESMART recorder.....................................................................29
5.1.2 Patient cable..............................................................................30
5.1.3 Recorder bag...............................................................................30
5.2 Maintenance..........................................................................................30
6 Service life time............................................................................................... 32
7 Operation control and error handling...................................................................33
7.1 Operation testing...................................................................................33
7.2 Main sources of error..............................................................................33
8 Technical specifications.....................................................................................35
8.1 EMC guidance and manufacturer's declaration.............................................37
9 Accessories and consumables..............................................................................40
TELESMART-H/P - Holter-ECG-Recording System - version D

Page 7
9.1 Accessories...........................................................................................40
9.2 Consumables.........................................................................................40
TELESMART-H/P - Holter-ECG-Recording System - version D

Seite 8 Application and Function
1 Application and Function
1.1 General
The PADSY-Holter Holter-ECG system consists of a TELESMART recorder with a
Memory card, a recorder bag, a colour-coded patient cable to allow connection of
individual ECG channels, and the PADSY-Holter evaluation software. The
TELESMART-H recorder is used for ambulance or stationary recording of 2 to 3
channel Holter-ECGs.
The TELESMART recorder is a device for the recording of 2-channel (TELESMART-H
and -P) ans 3-channel Holter-ECGs of ambulatory and stationary patients.
The TELESMART-H recorder is also equipped with a connection to automatically
recognize the pacemaker voltage in channel B.
The TELESMART-H patient cable connected to the recorder allows a surface ECG to be
taken using Holter-ECG electrodes and, after an analog to digital conversion, to be
stored in the TELESMART-H recorder on the memory card.
The TELESMART-H recorder is applied by doctors and medical professionals, who
either carry the TELESMART-H in its recorder bag during their everyday activities, or
leave it stationary to make single recordings up to 7 days in length.
Once a recording is made, the memory card is removed from the recorder and placed
in a card-reader. The card-reader transfers the saved ECG data to the PC to be
evaluated using the PADSY-Holter evaluation software.
The recorder as part of the Holter-ECG System PADSY-Holter conforms to all relevant
parts of standard EN 60601-2-47 and therefore fulfills all performance specifications
as required by this standard.
Further hints and information for the intended use and performance specifications
can be found within the instructions for use for PADSY-Holter and PADSY and within
the appendix.
1.1.1 Variants
TELESMART can be ordered with modified functional specifications. Functional
specificaions of indiviual recorder variants can be found in chapter “Technical
Specifications”.
!TELESMART-P is not intended for use with pacemaker patients. For examination of
patients, who carry an implanted pacemaker device, please use a TELESMART-H
recorder.
TELESMART-H/P - Holter-ECG-Recording System - version D

Application and Function Page 9
1.2 Sa ety
This user's manual forms an integral part of the TELESMART-H Holter-ECG recorder
system, and must therefore be kept in the vicinity of the product at all times.
Precise compliance with the user's manual is an essential condition for normal use,
correct application, and thus safety of patients and users.
!The TELESMART Holter-ECG recorder system must only be used by personnel
who, because of their training, knowledge and practical experience, are inclined
to use the system correctly.
!The user is responsible to ensure that, before each use, the unctional sa ety
and operating condition of the equipment is checked.
!System components, in particular electrodes and electrode leads, may only be
applied to patients who have healthy skin.
!
The TELESMART recorder is a type BF appliance, with increased voltage spike
protection. t is not intended for direct application to the heart. The signal
inputs are not protected against the discharge of a defibrillator on the patient.
Be ore de ibrillation events, the electrodes must be removed rom the
patient.
!
Please note that the conductive sections of the electrode cables must not be
allowed to contact any electrical equipment, electrical sockets, conductive, or
grounded objects. Check that the electrodes are sa ely separated rom all
electrical devices and sockets before the patient is connected to the recorder
via the electrode cables.
!
Magnetic and electrical fields can influence the recorder function. Ensure that
during operation, all non-Medset equipment operating in the vicinity complies
with the relevant EMC requirements. This equipment may not be operated
around x-ray equipment, tomographs, etc., because these devices are may
emit a higher level of electromagnetic interference, corrupting the
measurements.
!These products are not intended or operation in explosion hazard
designated areas (resulting from anaesthetic, skin cleaning or disinfectant
substances) or in areas with flame promoting atmospheres (as a result of >25%
oxygen in the ambient air or nitrous oxide) in rooms used for medical purposes.
!Only use disposable or rechargeable batteries that are specified in this user’s
manual. When changing batteries, pay close attention that they are installed
TELESMART-H/P - Holter-ECG-Recording System - version D

Seite 10 Application and Function
with the correct polarity (printed on the battery).
!Before storing the equipment for prolonged periods without use, remove the
disposable or rechargeable battery to prevent discharge and resulting
equipment damage.
!
Patient safety, maintenance of equipment function and optimum interference
immunity are ensured exclusively with original Medset accessories or with the
accessories and consumables recommended by Medset. Medset cannot guarantee
the safe and accurate operation of equipment used with different accessories
and consumables.
!
The device must never be modified and must always be operated according to
the instructions in this user’s manual. Maintenance and repairs may only be
performed by Medset customer service. Repairing the equipment in any other
way absolves Medset from any responsibility for the safety, reliability, or
functionality of the equipment.
!f TELESMART-H/P is operated using lithium-batteries, only batteries according
to D N EN 60086-4 may be used. Otherwise there is an increased risk of
hazardous excessive heat generation.
!Liquids must never penetrate the interior of electrical equipment. f liquid has
entered the recorder, it must be returned to Medset customer service for testing
before it is used again.
!
Dispose of packaging material appropriately. Ensure that it is out of reach of
children.
Disposal of the equipment and accessories at the end of its life must be
performed according to local electronics disposal regulations, or by sending the
old equipment to Medset at the customer’s cost, where Medset will dispose of
the equipment appropriately.
!Please do not wind the patient cable around the recorder. This dramatically
shortens its service life.
!Please avoid to exceed the maximal application time o disposable ecg
electrodes, as specified by the manufacturer.
!Please instruct the patient to consult a physician in case of appearance o skin
irritation.
TELESMART-H/P - Holter-ECG-Recording System - version D

Application and Function Page 11
!
f the product documentation as delivered with the system is partly damaged,
unreadable or by any other reason not accessible by the user of the system, the
documents must be ordered at Medset for replacement and made available to the
user, in order to ensure safe operation. This also includes information as given
on the name plate of the TELESMART recorder.
TELESMART-H/P - Holter-ECG-Recording System - version D

Seite 12 Controls and Components
2 Controls and Components
2.1 Recorder
a LCD display
b Marker key
c Menu key
d Battery compartment
e Plug socket and patient cable
2.2 Marker and menu keys
These keys are found on the recorder faceplate, and allow menu navigation.
Marker Key
The marker key is the large button below the LCD display on the TELESMART-H
recorder faceplate. During the ECG recording, the patient can use this button to
mark specific events that will be saved to the memory card and will be visible on the
ECG after it has been evaluated using the software. To do this, the patient must
briefly press the key until a signal tone is heard.
Before the recording starts, the marker key acts as an additional menu button, and
allows the selection and confirmation of menu options (see [menu] button section).
Menu Key
The menu key allows the selection and confirmation of menu options.
This keys function is displayed on the LCD screen.
TELESMART-H/P - Holter-ECG-Recording System - version D
a
b
d
e
cc

Controls and Components Page 13
2.3 Optical and acoustic signals
Optical Signals
All commands and error messages that occur during recorder operation are dis-
played.
Acoustic signals
System start (boot loader)
After „Medset Logo“ appears
System test successful
Recorder start successful
Keystroke
Marker key
Error message
Warning
TELESMART-H/P - Holter-ECG-Recording System - version D

Seite 14 Equipment startup
3 Equipment startup
The TELESMART-H recorder is shipped in an operable condition, and can be started
without performing any special commissioning procedures.
Before the first startup, it is recommended that a brief check be performed, as
described in the „Operation testing“ section.
3.1 Special hints or setting up the device with MacOS X
Please note, that on newer MacOS X operating systems for any device which is set-up for
Bluetooth, a connection code must be typed in. For the TELESMART recorder the
connection code “0000” must be used.
TELESMART-H/P - Holter-ECG-Recording System - version D

Operation Page 15
4 Operation
4.1 ECG recording setup
4.1.1 Recorder setup
1. Ensure that the memory card is inserted into the TELESMART-H recorder.
2. nsert the disposable or rechargeable battery in the battery compartment.
Pay attention to the polarity of the battery.
3. Once the battery (disposable or rechargeable) is inserted, the TELESMART-H
recorder turns on automatically.
4. The recorder then performs a self-test, which ensures proper battery volt-
age and memory card present. After turning on the recorder the state of
charge of the battery is displayed by a battery symbol on the right side of
th recorder display.
!A low-voltage warning will occur if the battery voltage is insufficient. Replace or
recharge the batteries and insert the fresh batteries into the recorder.
5. After several seconds of self-test, the recorder responds with the „Medset
Medizintechnik GmbH“ logo. During the system start, a progress bar may
be seen at the lower edge of the screen.
6. f something not described above occurs, please see chapter 2 and 6 in
this user’s manual.
Please instruct your patient about how to handle the TELESMART recorder. f your
patient will use the recorder at home, please hand out a copy of “TELESMART – n-
formation for Patients”. A template of this document you can find on your PADSY-
DVD.
4.1.2 Skin preparation
The role that skin preparation plays in yielding a good ECG recording is often under-
estimated.
Male patients, even those that have only light hair growth in the electrode applica-
tion zones, must still have these areas shaved carefully.
Using the preparation cream recommended by Medset, a very low electrical contact
resistance is achieved between the skin and electrode during cleaning and degreas-
ing of the skin.
Alcohol or rubbing compound is not suitable for skin preparation. Using these sub-
stances will clean and degrease the skin, but will not lower the skin’s contact resis-
tance.
TELESMART-H/P - Holter-ECG-Recording System - version D

Seite 16 Operation
The preparation cream should be sparingly applied to the skin using a gauze pad at
the electrode positions, and rubbed in a circular motion on the skin for approxi-
mately 5 seconds.
Cotton batton is not suitable, because it applies too much cream to a small area.
4.1.3 Electrode selection
The TELESMART-H recorder was validated for use with the following electrodes:
•ARBO H92SG as standard electrodes (only suitable for 24 hour use)
•Medicotest BlueSensor VL-00-S, that prevents the movement of the electrode
by the cable and vice versa.
•3M Red-Dot Electrodes (type 2560): The manufacturer specifies a maximal
application time of up to 5 days.
Electrodes that have been stored too long in the open, stored too hot or too cold,
or have passed their expiry date should not be used.
TELESMART-H/P - Holter-ECG-Recording System - version D
Operation Page 17
4.1.4 Electrode application
To yield reproducible recordings without artefacts, the optimal application of the
electrodes is necessary.
Attach the electrodes to the electrode leads before they are stuck to the skin. This
avoids applying hard pressure to the patient’s body.
For women with large breasts, the electrodes should either be stuck to the breasts
(small amplitude) or directly under them (sweat and artefacts through pressure).
The following cables may be used:
•Patient cable 2 or 2L, TELESMART-H or -P (2 channel ECG with 5 electrodes)
•Patient cable 3, TELESMART-H (3 channel ECG with 7 electrodes)
•Patient cable M, TELESMART-H (3 channel ECG with 3 electrodes)
The placement of electrodes is exploratory. Based on the position and condition of
the heart, modifications to the suggested electrode application can be made to in-
crease the signal quality.
!Please always connect the patient cable to your TELESMART recorder be ore you
apply the cable with electrodes to the patient!
For removal of the recorder from the patient, please disconnect first the electrodes
from the patients body and then remove the electrode from the cable by holding the
electrode-knob with one hand and pulling gently at the electrode with the other.
Please do not pull at the electrode cable. Doing so will shorten the life-time of the
cable significantly.
For changing the patient cable, please open the battery compartment, remove the
battery and open the connection of the patient cable to the recorder by gently
pulling the plug. Please do not pull the cable! Now you can connect the desired
patient cable with the recorder and re-assemble the device.
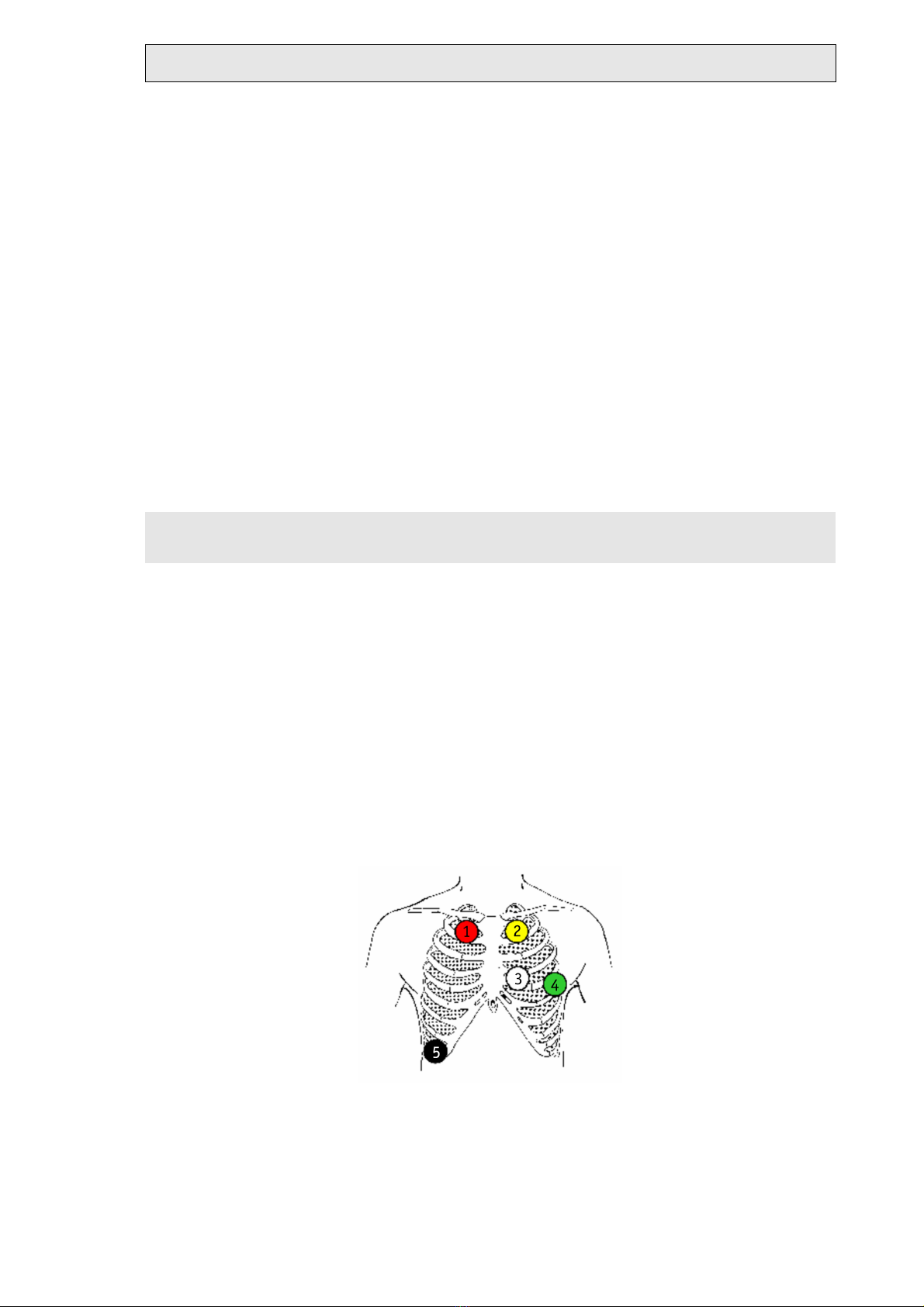
Electrode application or TELESMART patient cable 2, 2L TELESMART-H or -P:
Channel A modified V5:
+ green (4) Left fifth intercostal space in the anterior axillary line
- red (1) Right clavicle on sternum border
TELESMART-H/P - Holter-ECG-Recording System - version D
Cable 2: red (1), yellow (2), white (3),
green (4), black (5)
Seite 18 Operation
Channel B modified V2:
+ white (3) Left fourth intercostal space at sternal border
- yellow (2) Left clavicle at sternal border
Ground (reference potential):
black (5) right costal arch in the anterior axillary line
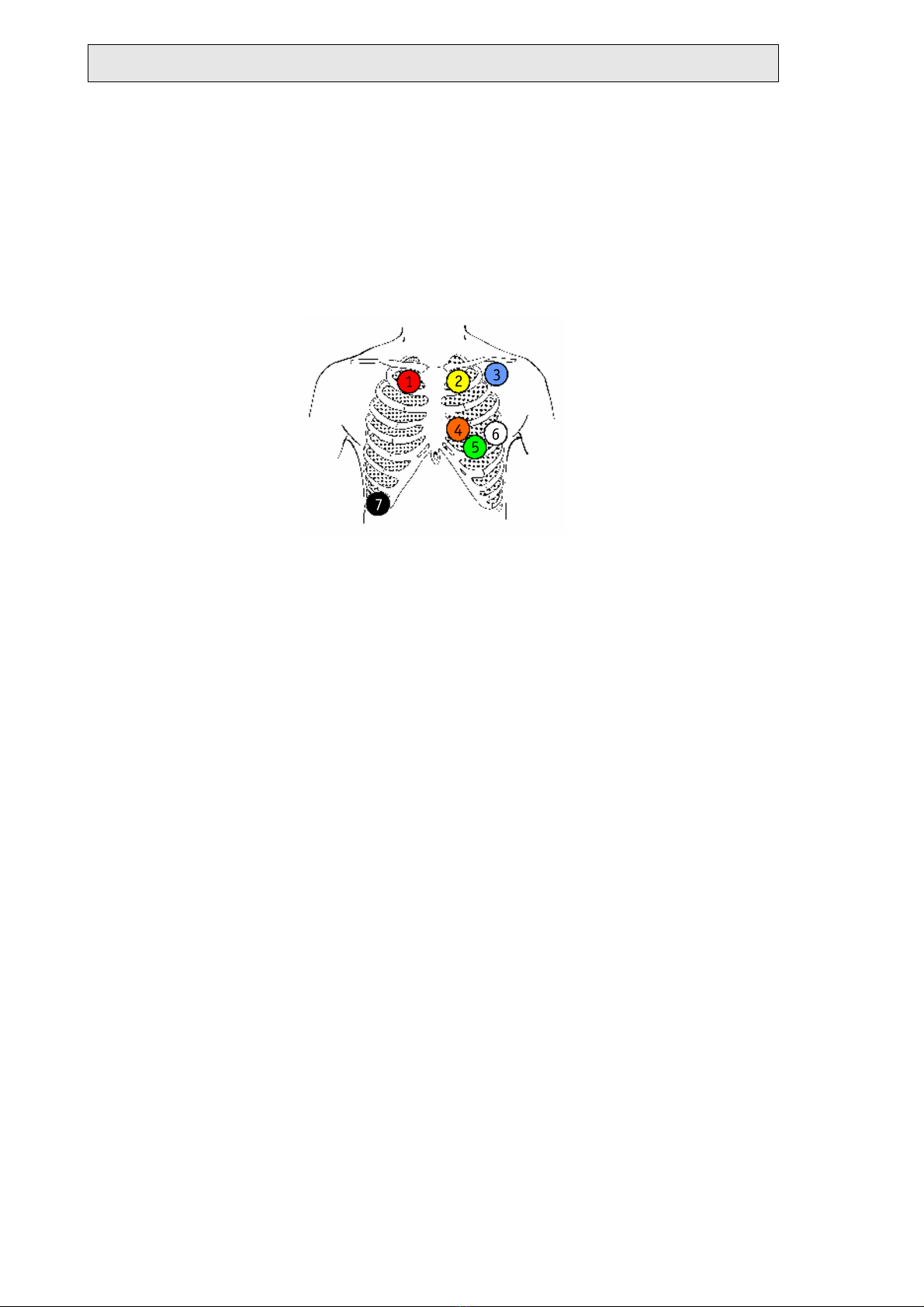
Electrode application or patient cable 3, TELESMART-H
Channel A modified V4:
+ green (5) Left fifth intercostal space in the midclavicular line
- red (1) Right clavicle at sternal border
Channel B modified V6:
+ white (6) Left fifth intercostal space in the anterior axillary line
- yellow (2) Left clavicle at sternal border
Channel C modified V2:
+ orange (4) Left fourth intercostals space at sternal border
- blue (3) Left clavicle in the anterior axillary line
Ground (reference potential):
black (7) Right costal arch in the anterior axillary line
Electrode application or patient cable M, TELESMART
TELESMART-H/P - Holter-ECG-Recording System - version D
Cable 3: red (1), yellow (2), blue (3),
orange (4), green (5), white (6), black
(7)

Operation Page 19
Channel A modified Vr5:
+ white (2) - red (1)
Channel B modified V5:
+ green (3) - red (1)
Channel C modified CC5:
+ green (3) - white (2)
Green (3): Left fifth intercostal space in the anterior axillary line
Red (1): On the sternum, level with the first intercostal space
White (2): Right fifth intercostal space in the anterior axillary line
TELESMART-H/P - Holter-ECG-Recording System - version D
Cable M: red (1), white (2), green (3)
Seite 20 Operation
4.2 Electrode arrangement or patients with pacemakers (TELESMART-H)
Using the TELESMART recorder (TELESMART-H, only) with PADSY-Holter software al-
lows the recognition and analysis of pacemaker pulses to be performed. Pulses of
uni and bi polar, one and two chamber pacemakers will all be recorded and recog-
nized.
Pacemaker pulse recognition occurs on channel B for all recorders. The high sample
frequency of the recorder means that the pacemaker pulses will be marked on the
real-time ECG recording at virtually the exact time point where they occurred. The
events are marked on the ECG with a red marker element. Please refer to the PADSY-
Holter software user’s manual for more detail.
Please note, that patient cable “M” is not intended or use with pacemaker pa-
tients.
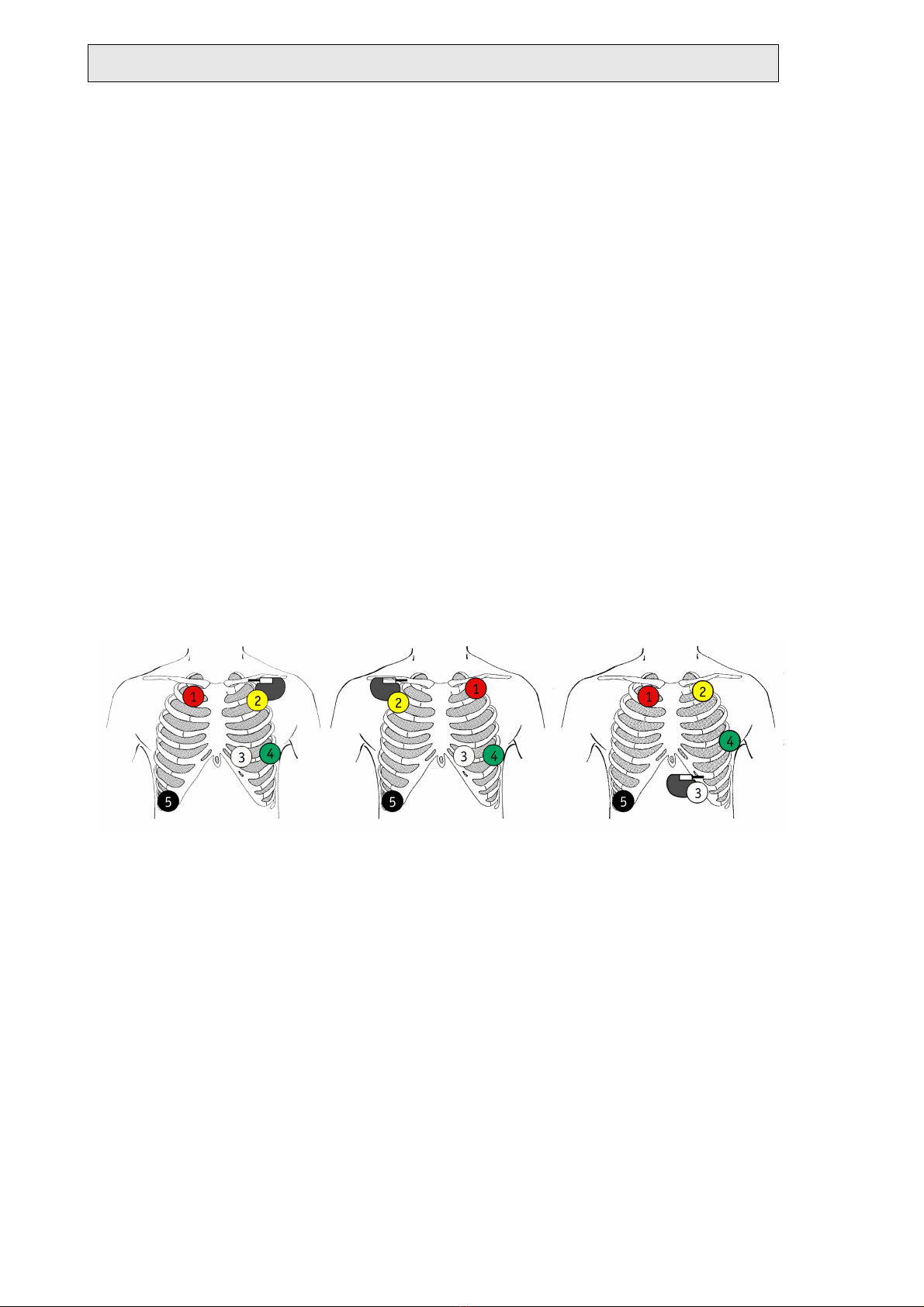
4.2.1 Patients with unipolar pacemakers
For unipolar pacemakers, the electrode array must be positioned according to the
pacemaker location. To accomplish this, the electrodes of channel B that lie closest
to the implanted pacemaker in the standard electrode configuration should be posi-
tioned directly over the pacemaker.
The following figures give examples for electrode arrays in channel B that are set up
for unipolar pacemakers.
Channel A: red (1)/green (4) electrodes
Channel B (pm): yellow(2)/white (3) electrodes
Ground (reference): black electrode (5)
TELESMART-H/P - Holter-ECG-Recording System - version D
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











