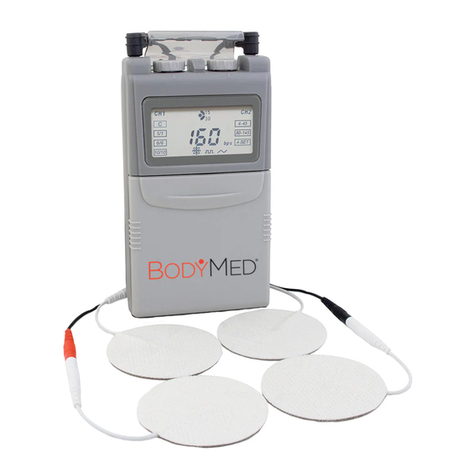
BodyMed ZZAEV820OTC User manual

ZZAEV820OTC
PAIN RELIEF SYSTEM
V1.0
Manufactured for:
BodyMed
Hudson, Ohio 44236
1.866.528.2152
C
M
Y
CM
MY
CY
CMY
K
820.ai 1 2015/5/27 下午 01:30:16820.ai 1 2015/5/27 下午 01:30:16

1
INDEX
Chapter Contents Page
Index ....................................................................................1
1. Pain Relief System .............................................................2
2. Important Safety Instructions ............................................2
3. Package Contents ..............................................................5
4. Parts Of Stimulator .............................................................6
5. How Your TENS Works. ......................................................7
6. Conditions That May Affect Your Pain Relief System ....7
7. Quick Operation Guide ......................................................7
8. Attachment Of Electrode Lead Wires ................................8
9. Lead Wire Maintenance ......................................................8
10. Electrode Options ...............................................................9
11. Electrode Placement ...........................................................9
12. Tips For Skin Care...............................................................9
13. Application Of Re-Usable Self Adhesive Electrodes........10
14. Operating Instructions........................................................12
15. Battery Information .............................................................14
16. Maintenance, Transportation And Storage Of
TENS Device........................................................................16
17. Safety-Technical Controls ..................................................16
18. When To Order New Electrodes ........................................17
19. Warranty ..............................................................................17
20. Replacement Parts .............................................................17
21. Conformity To Standards....................................................17
22. Trouble shooting Guide ......................................................18
23. Stimulator Technical Specications ..................................19

32
Chapter 1: PAIN RELIEF SYSTEM
Intended Use
The ZZAEV820OTC Pain Relief System is intended for temporary
relief of pain associated with sore and aching muscles in the lower
back due to strain from exercise or normal household and work ac-
tivities.
About TENS Technology
Transcutaneous Electrical Nerve Stimulation is an approved non-in-
vasive drug-free method of lower back pain management. It relieves
pain by sending small electrical impulses through electrodes placed
on the skin to underlying bers. TENS is believed to work by two
different mechanisms. First, electrical stimulation of the nerve bers
can block a pain signal from being carried to the brain. If the signal
is blocked, pain is not perceived. Secondly, the body has its own
mechanism for suppressing pain. It does this by releasing natural
chemicals called endorphins in the brain which act as analgesics.
TENS may activate this mechanism. By effectively managing pain
without drugs, TENS allows many people with lower back pain con-
ditions to resume daily activity.
Chapter 2: IMPORTANT SAFETY INFORMATION
Read instruction manual before operation. Be sure to comply with
all “CAUTIONS” and “WARNINGS” in the manual. Failure to follow
instructions can cause harm to user or device.
CAUTION: Indicates matters in which bodily harm or material dam-
age can occur.
WARNING: Indicates what you cannot do with device.
Please read the following information carefully before using this Low
Back Pain Relief System.
WARNING: DO NOT USE THIS SYSTEM IF ANY OF THE
FOLLOWING CONDITIONS ARE PRESENT.
• Do not use this System if you have a cardiac pacemaker,
implanted debrillators or any other implanted metallic or
electronic device.
• Do not use this System if you have undiagnosed chronic pain.
• If you are under the care of a physician, consult with your
physician before using this System.
• Do not use during pregnancy.
• Do not place the electrodes on or close to your heart.
• Do not place the electrodes around or close to your neck. Do not
apply stimulation over the neck.Severe spasm of the muscles
may occur and the contractions may be strong enough to close
the airway or cause difculty in breathing. Stimulation over the
neck could also have adverse effect hearing or blood pressure.
• Do not apply stimulation across the chest because the
introduction of electrical current into the chest may cause rhythm
disturbances to the heart.
• Do not place the electrodes on or around your head. The effects
of stimulation of the brain are unknown.
• Do not use this System while sleeping.
• Do not use if you feel numbness.
• Do not use this System in or close to water.
• Use the electrodes only on normal, healthy, clean and dry skin.
Do not use the electrodes on open wounds or rashes, or over
swollen, red, infected or inamed skin.
• If you have ever had back surgery, consult your physician before
using this System.
• If you have had medical or physical treatment for your muscle
pain, consult with your treatment provider before using this
System. You should contact your physician prior to using the
System following recent surgical procedures. Stimulation may
disrupt the healing process.
CAUTION: DISCONTINUE USE AND CONSULT YOUR PHYSI-
CIAN IF ANY OF THE FOLLOWING CONDITIONS APPLY TO YOU.
• If you have suspected or diagnosed heart problems.
• If you have suspected or diagnosed epilepsy.
• If you have a tendency to bleed internally following an injury.

54
• If areas of skin lack normal sensations, such as skin that tingles
or is numb.
• During menstruation.
• The long-term effects of this System are not known.
CAUTION:
• The System is intended for individual person use for the
temporary relief of lower back pain.
• The System is not effective for pain associated with Central Pain
Syndromes, such as headaches.
• The System is for pain caused by muscle soreness, and should
be placed only around muscles where pain originates on the
lower back pain area.
• The pain may indicate that you have some other health problem.
You should know the reason and source of your pain before
using this System. Do not solely reply on the treatment of this
System for pain.
• Some people may feel skin irritation or experience a very
sensitive feeling in the skin due to electrical stimulation. If this
occurs, stop using your System and consult your physician.
• Some people may feel skin irritation from the adhesive on the
self-adhesive electrodes. If this occurs, stop using your System.
• Minor redness at stimulation placement is a normal skin reaction.
It is not considered as skin irritation, and it will normally disappear
within 30 minutes after the electrodes are removed. If the
redness does not disappear after 30 minutes from the removal of
electrodes, do not use the stimulator again until after the
excessive redness has disappeared.
• Turn off the stimulator if the stimulation feels unpleasant or does
not provide pain relief.
• Keep your System out of the reach of children.
• Do not use this System when driving, operating machinery or
when swimming.
• The effectiveness of the System depends greatly on a person’s
individual physical condition. It may not always be effective for
every user.
• Clean any adhesive residue left on the skin with soap and water.
Do not use alcohol or solvent based cleaning products on your
skin.
• If skin under one of more electrodes feels irritated after using the
stimulator for a long period of time, use the stimulator for a
shorter period of time.
• Use your stimulator only with the electrodes, lead wire and
accessories recommended by the manufacturer.
• Before removing the electrodes, be sure to turn OFF both
power knobs to avoid unpleasant stimulation.
Adverse Reactions
• Skin irritation and burns under the electrodes have been reported
by some people who have applied electronic stimulators to their
skin.
SAVE THESE INSTRUCTIONS
Chapter 3: PACKAGE CONTENTS
Each ZZAEV820OTC pain relieve device comes complete with
standard accessories and the standard labels as given below:
DESCRIPTION Q’TY
1. 40 X 40 mm Adhesive Electrodes 4 pieces
2. Electrodes Leads 2 pieces
3. 9 V Battery, type 6F22 1 piece
4. Instruction Manual 1 piece
5. Carrying Case 1 piece

76
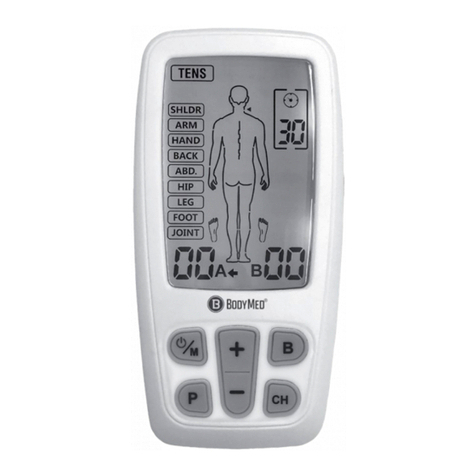
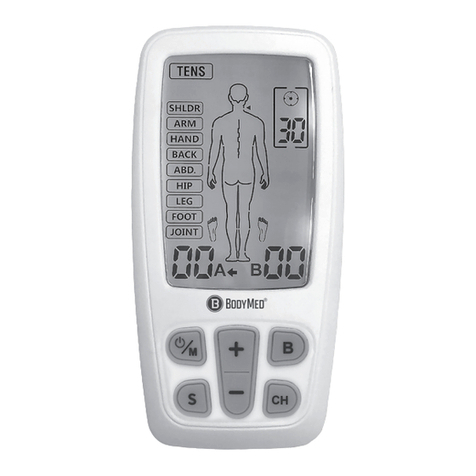
Chapter 4: PARTS OF STIMULATOR Chapter 5: HOW YOUR TENS WORKS
The main function of pain relief comes from the continuous stimula-
tion pulse generated from the dual channel stimulator. We can not
ensure that the pain relief is 100% effective for everyone.
Chapter 6: CONDITIONS THAT MAY AFFECT
YOUR PAIN RELIEF SYSTEM
Since the stimulator is a battery-operated electronic system, its
output performance and safety may be affected greatly in extreme
humidity. Therefore, it is very important to keep the stimulator dry to
ensure the safety and performance of the stimulator.
Chapter 7 : QUICK OPERATION GUIDE
Your may use the following simple operation steps to operate this
System.
Step 1. Insert the 9-volt battery into your stimulator
Step 2. Apply electrode electrodes to the skin
Step 3. Connect the lead wire
Step 4. Turn the stimulator “ON”, adjusting the amplitude
adjustment knobs to the desired stimulation strength
Step 5. Select the therapy mode
Step 6. Select the stimulation time
Step 7. Enjoy the benets
Step 8. Turn off stimulator
Step 9. Locking and unlocking the stimulator settings
Step 10. Viewing the therapy timer
For the details of operation, please see the following operating
instructions.
1. On/Off and Amplitude Control
2. On/Off and Amplitude Control
3. LCD
4. Timer Control
5. Mode Selector
6. Battery Strip
7. Lead Connector
8. Flip Top Cover
9. Belt Clip

98
Chapter 8: ATTACHMENT OF ELECTRODE
LEAD WIRES
The wires provided with the system insert into the jack sockets
located on top of the device. Holding the insulated portion of the
connector, push the plug end of the wire into one of the jacks (see
drawing); one or two sets of wires may be used.
After connecting the wires to the stimulator, attach each wire to an
electrode. Use care when you plug and unplug the wires. Jerking
the wire instead of holding the insulated connector body may cause
wire breakage.
CAUTION
Do not insert the plug of the patient lead wire into any AC power
supply socket.
Chapter 9: LEAD WIRE MAINTENANCE
Clean the wires by wiping with a damp cloth. Coating them lightly
with talcum powder will reduce tangling and prolong life.
Chapter 10: ELECTRODE OPTIONS
The electrodes are disposable and should be routinely replaced
when they start to lose their adhesive nature. If you are unsure of
your electrode adhesive properties, order replacement electrodes.
Replacement electrodes should be re-ordered through or on the
advice of your physician to ensure proper quality. Follow application
procedures outlined in electrode packing, to maintain optimal stimu-
lation and to prevent skin irritation.
Chapter 11: ELECTRODE PLACEMENT
The placement of electrodes can be one of the most important
paragraphs in achieving success with TENS therapy. It is important
that the physician experiments to determine optimum electrode
placement.
Chapter 12: TIPS FOR SKIN CARE
To avoid skin irritation, especially if you have sensitive skin, follow
these suggestions:
1. Wash the area of skin where you will be placing the electrodes,
using mild soap and water before applying electrodes, and after
taking them off. Be sure to rinse soap off thoroughly and dry skin
well.
2. Excess hair may be clipped with scissors; do not shave
stimulation area.
3. Wipe the area with the skin preparation your physician has
recommended. Let this dry. Apply electrodes as directed.
4. Many skin problems arise from the "pulling stress" from adhesive
patches that are excessively stretched across the skin during
application. To prevent this, apply electrodes from centre outward;
avoid stretching over the skin.
5. To minimize "pulling stress", tape extra lengths of lead wires to
the skin in a loop to prevent tugging on electrodes.
6. When removing electrodes, always remove by pulling in the

1110
direction of hair growth.
7. It may be helpful to rub skin lotion on electrode placement area
when not wearing electrodes.
8. Never apply electrodes over irritated or broken skin.
Chapter 13: APPLICATION OF RE-USABLE SELF
ADHESIVE ELECTRODES
Application
1. Clean and dry the skin at the prescribed area thoroughly with
soap and water prior to application of electrodes.
2. Insert the lead wire into the pin connector on the pre-wired
electrodes.
3. Remove the electrodes from the protective liner and apply the
electrodes rmly to the treatment site.
Removal
1.
Lift at the edge of electrodes and peel;
do not pull on the lead wires because
it may damage the electrodes.
2. Place the electrodes on the liner and
remove the lead wire by twisting and
pulling at the same time.
Care and Storage
1. Between uses, store the electrodes in the resealed bag in a cool
dry place.
2. It may be helpful to improve repeated application by spreading a
few drops of cold water over the adhesive and turn the surface
up to air dry. Over saturation with water will reduce the adhesive
properties.
Important
1. Do not apply to broken skin.
2. The electrodes should be discarded and re-ordered from your
physician when they are no longer adhering.
3. The electrodes are intended for single patient use only.
4. If irritation occurs, discontinue use and consult your physician.
5. Read the instructions for use of self-adhesive electrodes before
application.

1312
Chapter 14: OPERATING INSTRUCTIONS
1. Slide-on Cover:
A slide-on panel cover covers the controls for
selecting mode and adjusting settings. Your
medical professional may wish to set these
controls for you and request that you leave the
cover in place.
2. Power On/Off Switch and Intensity Controls:
If both controls are in the off-position, the device is switched off.
By turning the controls clockwise, the appropriate channel is
switched on and the indicator of power (CH1 or CH2) will reveal
on the LCD.
The current strength of the impulses transmitted to the electrodes
increases further when the control is turned clockwise.
To reduce the current strength or switch the device off, turn the
control counter clockwise to the required setting or off-position,
respectively.
The controls are protected by a cap to avoid unintentional change
of intensity.
3. Lead Connector
Connection of the electrodes is made with the two-lead
connector (lead wires). The device must be switched off before
connecting the lead wire. Both intensity controls must be at the
Off position. Electrodes must be pressed rmly on the skin.
4. Mode Control
There are 8 programs of option from P1 to P8. Press this bottom to
select a program desired.
5. Timer
The treatment time can be selected by pressing the “Timer” control.
There are ve settings available: 15, 30, 60 minutes and continue.
Press the “Timer” control to select a.
6. Low Battery Indicator
A low battery indicator will show up when
the battery is low. Replace battery when the stimulation can not
be felt.

1514
7. Check/Replace the Battery:
Over time, in order to ensure the functional safety of TENS,
changing the battery is necessary.
1. Make sure that both intensity controls
are switched to Off position.
2.
Slide the battery compartment cover and
open.
3. Remove the battery from the
compartment.
4. Insert the battery into the compartment.
Note the polarity indicated on the bat-
tery and in the compartment.
5. Replace the battery compartment cover
and press to close.
Chapter 15: BATTERY INFORMATION
PRECAUTIONS
1.
Remove battery if equipment is not likely to be used for some time.
2. Please recycle the used battery in accordance with domestic
regulation.
3. Do not throw the used battery into a re.
If you use rechargeable batteries, please follow the instructions.
RECHARGEABLE BATTERIES(NOT INCLUDED)
Prior to the use of a new unit, the rechargeable battery should be
charged according to the battery manufacturer's instructions. Before
using the battery charger, read all instructions and cautionary mark-
ings on the battery and in this instruction manual.
After being stored for 60 days or more, the batteries may lose their
charge. After long periods of storage, batteries should be charged
prior to use.
BATTERY CHARGING
(1) Plug the charger into any working 110 or 220/240v mains
electrical outlet. The use of any attachment not supplied with the
charger may result in the risk of re, electric shock, or injury to
persons.
(2) Follow the battery manufacturer's instructions for charging time.
(3)
After the battery manufacturer's recommended charging time has
been completed, unplug the charger and remove the battery.
(4) Batteries should always be stored in a fully charged state.
To ensure optimum battery performance, follow these guidelines:
(a) Although overcharging the batteries for up to 24 hours will
not damage them, repeated overcharging may decrease
useful battery life.
(b) Always store batteries in their charged condition. After a
battery has been discharged, recharge it as soon as possible.
If the battery is stored more than 60 days, it may need to be
recharged.
(c) Do not short the terminals of the battery. This will cause the
battery to get hot and can cause permanent damage. Avoid
storing the may accidentally batteries in your pocket or purse
where the terminals mayaccidentally come into contact with
coins, keys or any metal objects.
(d) WARNINGS:
1. Do not attempt to charge any other types of batteries in
your charger, other than rechargeable batteries made for
your charger. Other types of batteries may leak or burst.
2. Do not incinerate the rechargeable battery as it may
explode!

1716
Chapter 16: MAINTENANCE, TRANSPORTATION
AND STORAGE OF TENS DEVICE
1.
Non-ammable cleaning solution is suitable for cleaning the device.
Note: Do not smoke or work with open lights (for example, candles,
etc.) when working with ammable liquids.
2. Stains and spots can be removed with a cleaning agent.
3. Do not submerge the device in liquids or expose it to large
amounts of water.
4. Return the device to the carrying box with sponge foam to en-
sure that the unit is well-protected before transportation.
5.
If the device is not to be used for a long period of time, remove the
batteries from the battery compartment (acid may leak from used
batteries and damage the device). Put the device and
accessories in carrying box and keep it in cool dry place.
6.
The packed TENS device should be stored and transported under
the temperature range of -20℃~ + 60℃, relative humidity
20% ~ 95%, atmosphere pressure 500 hPa ~ 1060 hPa.
Chapter 17: SAFETY-TECHNICAL CONTROLS
For safety reasons, review the following checklist before using your
ZZAEV820OTC pain relief system
1. Check the device for external damage.
- Deformation of the housing.
- Damaged or defective output sockets.
2. Check the device for defective operating elements.
- Legibility of inscriptions and labels.
- Make sure the inscriptions and labels are not distorted.
3. Check the usability of accessories.
- Patient cable undamaged.
- Electrodes undamaged.
- Battery is not corroded
Please consult your distributor if there are any problems with device
and accessories.
Chapter 18: WHEN TO ORDER NEW ELECTRODES
The adhesive electrode electrodes are reusable. Electrodes should
be replaced when they lose their adhesive quality, or you sense a
change in stimulation sensation. To order new electrodes, see re-
placement Parts on page 17.
Chapter 19: WARRANTY
All ZZAEV820OTC TENS DEVICE models carry a warranty of one
year from the date of delivery. The warranty applies to the stimulator
only and covers both parts and labor relating thereto.
The warranty does not apply to damage resulting from failure to
follow the operating instructions, accidents, abuse, alteration or
disassembly by unauthorized personnel.
Chapter 20: REPLACEMENT PARTS
Contact your distributor to order replacement parts.
- Lead wires
- Electrodes
- Batteries
Chapter 21: CONFORMITY TO STANDARDS
The Stimulator is in compliance with:
• ANSI/AAMI ES 60601-1 – Safety of medical electrical
equipment – General safety.
• IEC 60601-1-2: EMC test
• ANSI/AAMI NS 4 – Transcutaneous Electrical Nerve
Stimulators

1918
Chapter 22: TROUBLE SHOOTING GUIDE
If you have a problem using your ZZAEV820OTC Pain Relief
System,review the following table to nd a solution.
Problem Solution
The stimulator will not turn on. Check the battery – be sure it is rmly in
place.Change the battery if necessary.
The stimulator turns on and Replace the battery.
then off quickly.
The stimulator is on, but there Check the lead wire for breakage.
is no stimulation. If broken, replace.
The display is frozen in some You are in “Lock” function now.The following
mode and the lock symbol steps will reset the unit to normal function.
appears. 1. With unit off, press and hold down the
TIME Button.
2. With the TIME button pressed, turn the
unit on.
3. Press the UP arrow once to turn the
“LOCK” symbol off.
4. Turn unit off.
5. Turn the unit on normally, it will back to
normal function.
The stimulator has a new 9-volt Please consult or return the device to your
battery, but will not turn on. dealer.
Chapter 23: STIMULATOR TECHNICAL
SPECIFICATIONS
Stimulator Technical Specications
Power Source: 9 Volt battery.
Wave Form: Asymmetrical Bi-Phasic Square Pulse
Dimensions: 110 x 62 x 27mm.
Weight: Approx. 142 grams (battery included)
Program Max. Phase Duration Rate Function Mode
P1 80mA 260μs 15Hz Constant
P2 80mA 260μs 100Hz Burst
P3 80mA 260μs 120Hz Constant
P4 80mA 260~150μs 2~100Hz Modulated
P5 80mA 260μs 100Hz Modulated
P6 80mA 260μs 80<->7 Hz Modulated
P7 80mA 260μs 120Hz Modulated
P8 80mA P1~P7 Cycle
Timer: 15, 30, 60 minutes, and continuous mode selectable.
Table of contents
Other BodyMed Medical Equipment manuals

BodyMed
BodyMed ZZRWAL03 User manual

BodyMed
BodyMed ZZACOMBO User manual

BodyMed
BodyMed ZZHP147 User manual

BodyMed
BodyMed zza350t User manual
BodyMed
BodyMed ZZAIF400 User manual

BodyMed
BodyMed ZZA250 User manual

BodyMed
BodyMed ZZA500 User manual
BodyMed
BodyMed ZZACOMBOBDPT2 User manual
BodyMed
BodyMed ZZACOMBOBDPT1 User manual

BodyMed
BodyMed ZZAN602 User manual