DISCLAIMER:
The techniques and procedures described do not represent all medically acceptable protocols, nor are they intended as a substitute for institutional
protocols or the physician’s experience and judgment in treating any specific patient. All available data, including the patient’s signs and symptoms and
other diagnostic test results, should be considered before determining a specific treatment.
The ZEPHYR VCB’s compression force is designed to automatically decrease by up to 40% in 3 hours as observed in laboratory testing.
CAUTION:
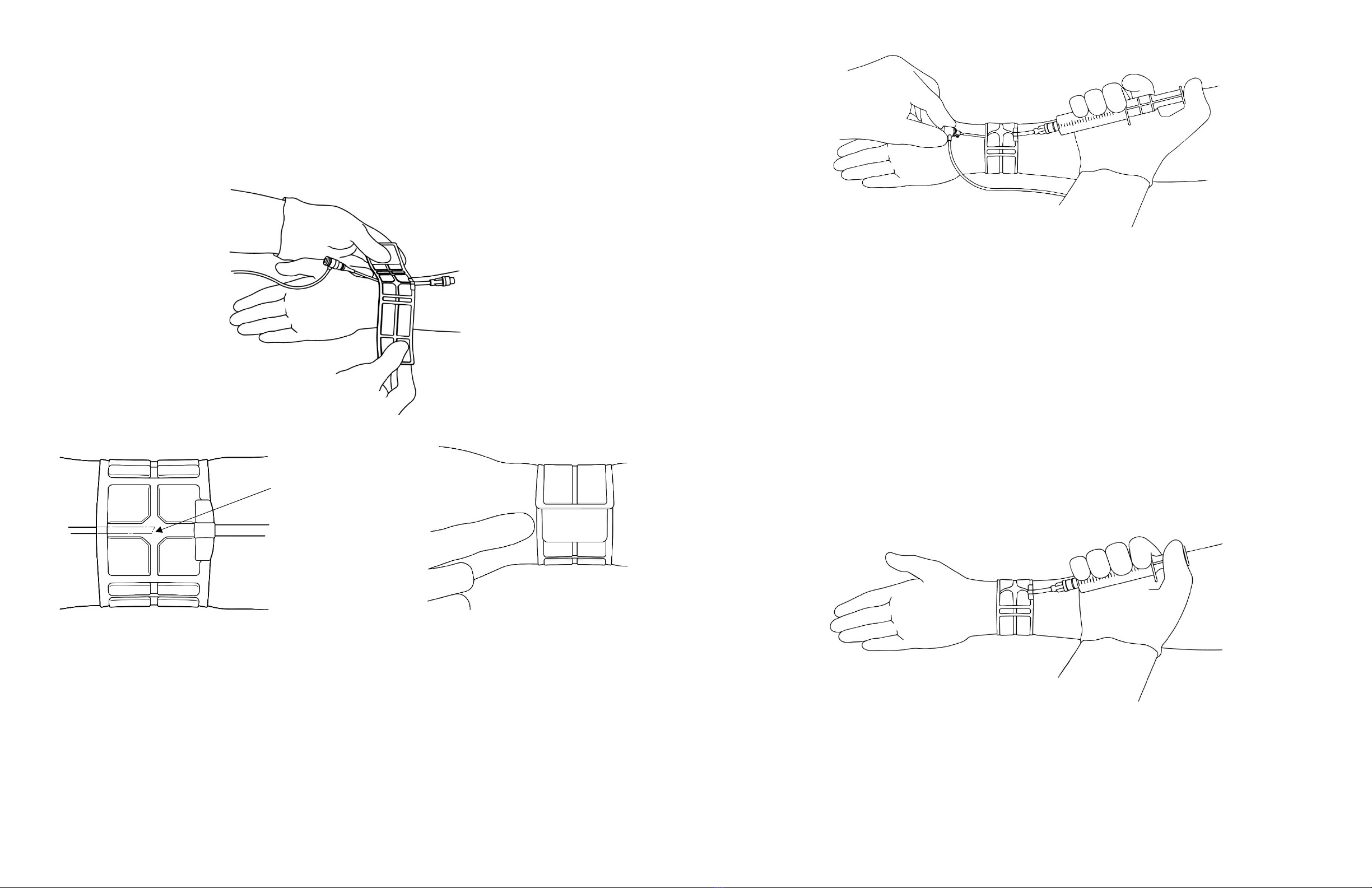
The ZEPHYR VCB must be applied by a physician, nurse or technician experienced with vascular procedures. The patient must be checked regularly
for arterial patency, bleeding, hematoma or thrombosis while the ZEPHYR VCB is in use.
The ZEPHYR VCB should be used only for hemostasis of a puncture site on a patient’s limb. Sterile or aseptic technique should be used.
PRECAUTIONS
• Sterility of package contents is not guaranteed if the individual packages are previously damaged or opened. Only new, sterile ZEPHYR VCBs taken from
factory-sealed pouches, should be used.
• The ZEPHYR VCB is a single-use, sterile device. Attempts to re-use or re-sterilize may result in device breakage or malfunction resulting in patient injury or
infection. Do not use if the package has been opened or damaged.
• Do not use alcohol, disinfectants, or any other liquids with the ZEPHYR VCB or on the patient while the ZEPHYR VCB is being applied. The ZEPHYR VCB
must be deployed onto a dry site.
• Federal (USA) law restricts this device to sale by or on the order of a physician.
• The ZEPHYR VCB deployment should be performed by physicians or physician-directed allied health care professionals with adequate training in the use of
the device.
• Use caution when applying compression with the ZEPHYR VCB, taking care not to overtighten it.
• Do not over-inflate the balloon. Over-inflation can result in balloon damage that compromises the performance of the ZEPHYR VCB.
• Only insert the nozzle of the syringe into the valve on the ZEPHYR VCB. Do not insert the nozzle of the syringe into a Luer connector or valve on a
sheath or any other device.
• Do not inject any liquids into the ZEPHYR VCB balloon.
• Use caution if deploying the ZEPHYR VCB onto a patient with uncontrolled hypertension or systolic pressure of more than 180mmHg.
• When operating the syringe, control the plunger by pressing on the end at all times.
• Ensure correct placement, alignment and securement of the ZEPHYR VCB.
• Patients should not be left unattended while the ZEPHYR VCB is in use.
• Do not leave the ZEPHYR VCB on for inappropriately long periods of time as tissue damage may occur.
• Do not apply if the circumference of the wrist at the puncture site is too large or small, exceeding the size range of the ZEPHYR VCB.
• Instruct the patient not to touch or bump the ZEPHYR VCB or move their hand or wrist during compression.
• Monitor the patient during the compression period for bleeding, hematoma or thrombosis, and to ensure proper deployment and patent distal blood flow
through the ulnar and radial arteries.
CONTRAINDICATIONS
• Patients with infection or other serious skin diseases at the site of puncture.
• Patients with an abnormal Allen test or radial pulse, or insufficient blood supply in the ulnar or radial arteries.
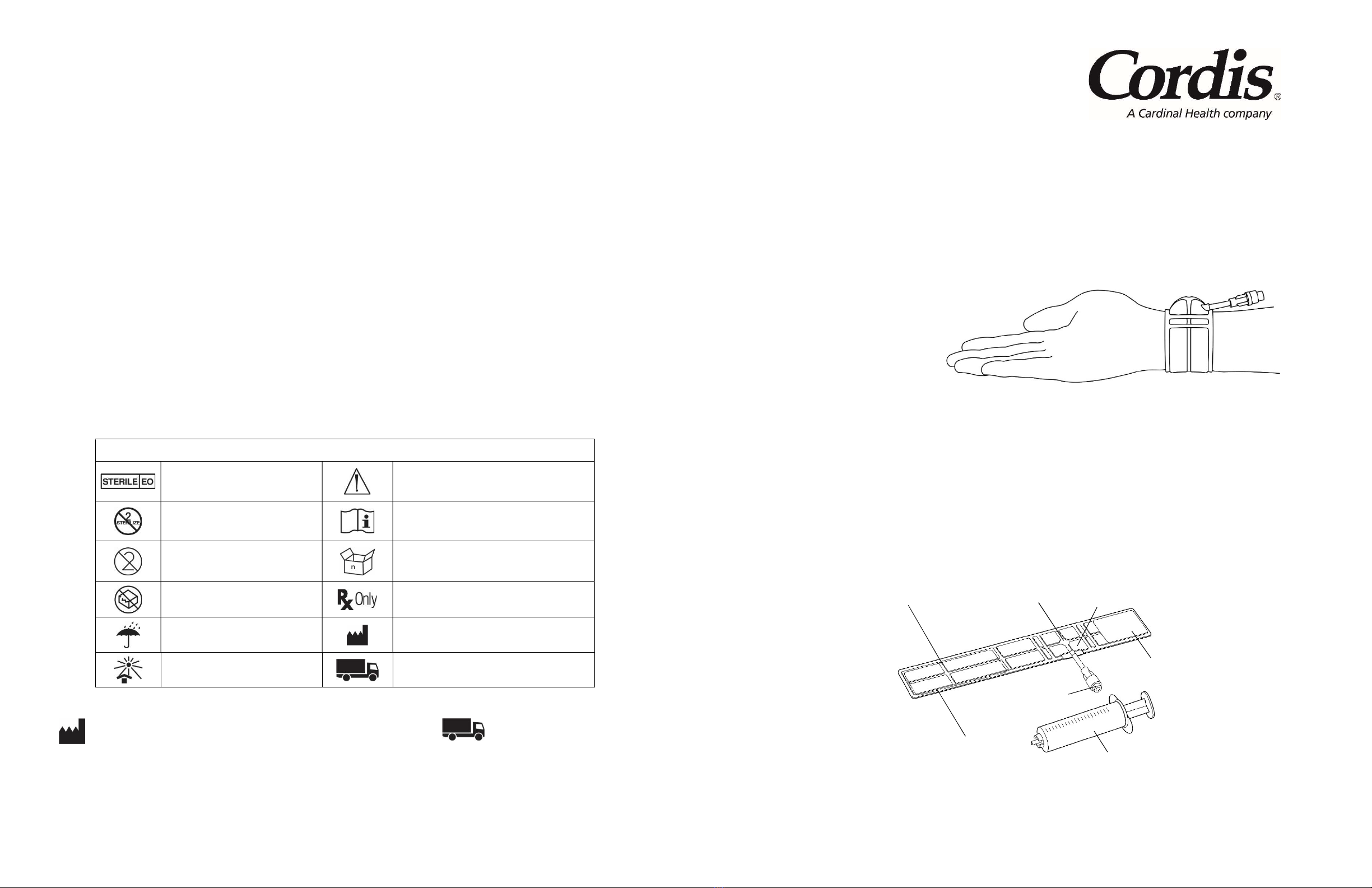
Explanation of Symbols
Sterilized with ethylene oxide gas
Caution
Do not resterilize
Consult instructions for use
Do not re-use
n units per box
Do not use if package is damaged
Caution: Federal (USA) law restricts this device
to sale by or on order of a physician
Keep dry Manufacturer
Keep away from sunlight Distributed by
Advanced Vascular Dynamics
4252 SE International Way, #F
Milwaukie, Oregon 97222 USA
Cordis Corporation
14201 North West 60th Ave
Miami Lakes, Florida 33014 USA
This product is not made with natural rubber latex, DEHP or other phthalates. The ZEPHYR VCB contains no user-repairable parts.
U.S. Pat. No. 9,427,239. Pats. Pending. ZEPHYR is a registered trademark of Advanced Vascular Dynamics, a Semler Technologies company.
© 2018, Cardinal Health. All Rights Reserved. 061-9100-06-C, 8/2018
User Guide
ZEPHYR Vascular Compression Band
for Radial Artery Hemostasis
INDICATION FOR USE
The ZEPHYR Vascular Compression Band (VCB) is indicated for use by medical professionals to promote hemostasis following a catheterization or other
puncture into a blood vessel in a patient’s arm or leg, including: radial, brachial, dorsalis pedis, or tibial blood vessels, arterial or venous line or sheath removal,
hemodialysis, and in anticoagulation therapy.
NOTE: This User Guide is provided for the radial artery hemostasis specifically.
DESCRIPTION
The ZEPHYR VCB applies compression over a blood vessel in a patient’s arm to help achieve patent hemostasis. Each VCB includes the following:
Figure 1: Device Schematic
Model 190101 REGULAR Size 9.75” / 25 cm long
Model 190102 LARGE Size 11.75” / 30 cm long (with an extender not shown in Figure 1)
* NOTE: ANY LUER SYRINGE MAY BE USED TO INFLATE OR DEFLATE THE ZEPHYR VCB.
strap
indow radial balloon
radial balloon valve
“loop” closure
(bottom side)
20 ml syringe*