Deesse Pro Express User manual















Table of contents
Popular Personal Care Product manuals by other brands
A/V Stim
A/V Stim MindSpa Instruction and Use Guide
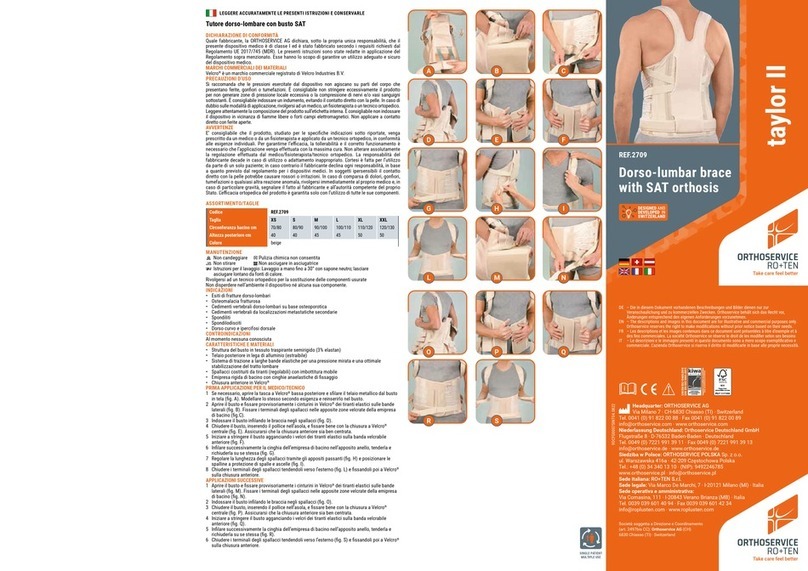
ORTHOSERVICE RO+TEN
ORTHOSERVICE RO+TEN taylor II quick start guide
Fleurco
Fleurco HALO PLUS MHAP1836 Installation and user guide

Aspen
Aspen PEAK SCOLI Patient Handbook

Lumie
Lumie Desklamp user guide

Philips AVENT
Philips AVENT AVENT SCF145/06 Specification sheet
Panasonic
Panasonic EH2511 operating instructions
Verilux
Verilux VM03 manual

Bestron
Bestron ASB350P instruction manual

NeuroSky
NeuroSky MindWave Mobile Pairing and Activation Guide

Ventec Life Systems
Ventec Life Systems VOCSN quick start guide

HoMedics
HoMedics spa REFLECTIVES M-6011 Instruction manual and warranty information

Performance Health
Performance Health 09 138 3561 manual

Fellowes
Fellowes 80309 owner's manual

sensiplast
sensiplast PRO COMFORT Instructions for use
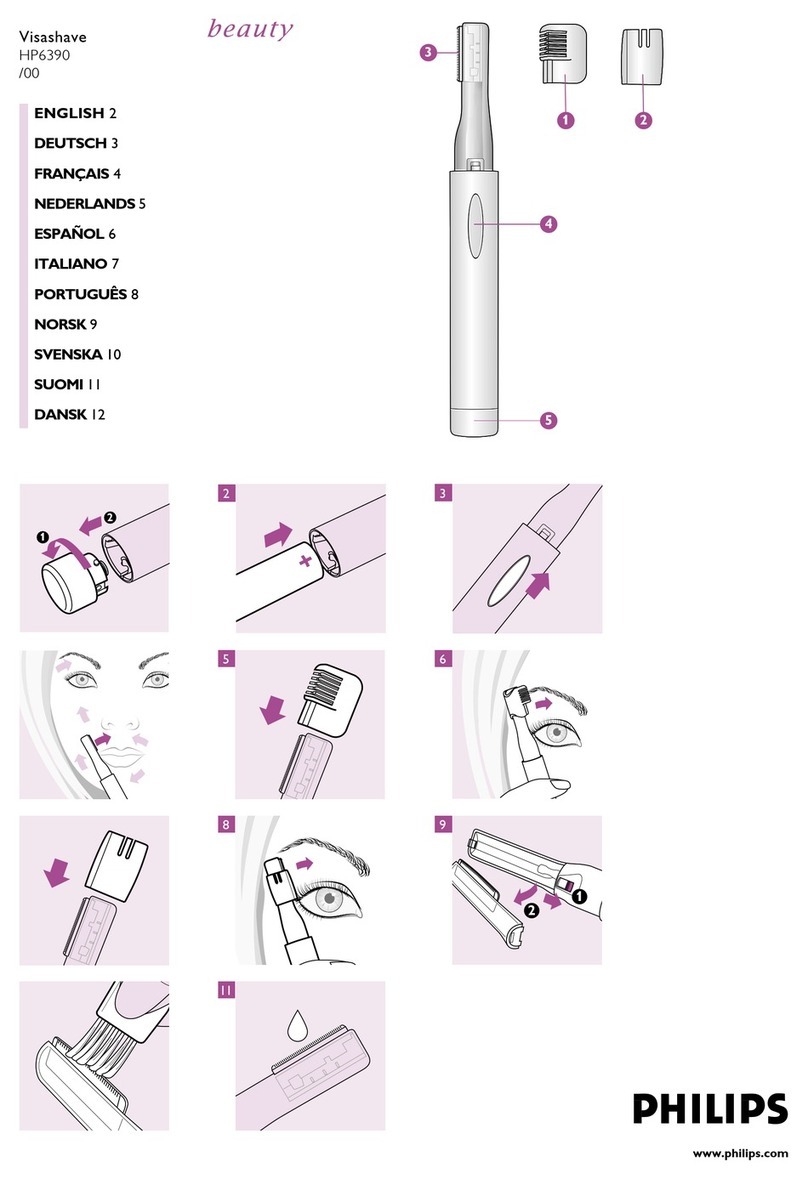
Philips
Philips Visashave HP6390/00 Instruction

Panasonic
Panasonic EH-SA31 operating instructions

Vulpes Electronics
Vulpes Electronics Heated mittens Welcome guide