Dermalux FlexMD User manual

USER GUIDE
dermaluxled.com
PL-027 (Rev 7)

Aesthetic Technology Limited
2 3
PL-027 (Rev 7) PL-027 (Rev 7)
Contents
1 Introduction 4
2 Important Safety Notice 5
3 Intended Use 5
4 Device Description 6
5 Device Components 7
6 Flex MD Quick Start Guide 8
7 Indications For Use 10
8 Precautions and Contraindications 11
9 Contraindications 12
10 Warning Signs Associated with Photosensitivity 13
10.1 Other substances known to increase the risk of sensitivity 13
10.2 Recognising Photosensitivity 13
11 Pregnancy 14
12 Precautions 14
13 Known Risks 14
14 Adverse Event Reporting 15
15 General Safety Warnings 16
16 To Reduce The Risk Of Damaging The System 18
17 Eye Safety 18
18 Receiving the Dermalux® Flex MD 19
19 Using the Device 20
19.1 Assembly Guide 20
19.2 Setup and First Use 23
19.3 Startup & Shutdown 24
19.4 Icons Explained 25
19.4.2 Detailed Software Walk Through 26
19.4.3 Red 633nm Treatment 28
19.4.4 Blue 415nm Treatment 29
19.4.5 NIR 830nm Treatment 30
19.4.6 Blue & Red Treatment 31
19.4.7 Blue & NIR Treatment 32
19.4.8 Red & NIR Treatment 33
19.4.9 Tri-Wave Treatment 34
20 Cleaning Instructions 35
21 Maintenance 35
22 Service Requirements 35
23 Storage & Transit Conditions 36
24 Operating Conditions 36
25 Disposal 37
26 Technical Specification 38
26.1 Electromagnetic Guidance 39
27 Labelling 40
28 Basic User Approach 41
29 System Warranty 42
30 Fault Reporting 44
31 Troubleshooting 45
32 Manufacturers Declaration 47
Aesthetic Technology Limited
4 5
PL-027 (Rev 7) PL-027 (Rev 7)
Thank you for choosing the Dermalux® Flex MD LED Phototherapy system. This User Guide covers
the operational and technical aspects of the system.
For Assembly Instructions, please refer to the Dermalux Flex MD Assembly Guide (PL-028).
Find instructions in Section 19 of this guide.
For suggested treatment protocols and indications for use, please refer to the Dermalux® Flex MD
Treatment Protocol Chart (PL-025) supplied with your system.
For a Quick Start Guide refer to document (PL-029). Find instructions in Section 6 of this guide.
For assistance setting up the equipment please refer to Page 20, Section 19, for any further
information please contact Aesthetic Technology Ltd, or your local Dermalux representative,
details found on page 42.
Online materials available at: learn.dermaluxled.com
1. Introduction
WARNING! Do not stare at operating LED Array. May be harmful to the eyes!
WARNING! Do not connect or disconnect the power cable or controller when power is live to the
device. Ensure power is off at all times when not in use.
WARNING! Do not connect any other power supply other than the original supplied with the
equipment at time of purchase.
WARNING! Risk of small parts, keep out of reach of children. Do not place objects in mouth.
Risk to inhalation or swallowing parts if damaged.
WARNING! Use of this equipment adjacent to or stacked with other equipment should be
avoided as it could result in improper operation. If such use is necessary, this equipment and the
other equipment should be observed to verify that they are operating normally.
3. Intended Use
The Flex MD is a Light Emitting Diode (LED) Phototherapy system that emits low-level
light energy from the visible and infrared region of the light spectrum.
It is indicated for the treatment and management of a wide range of cosmetic,
dermatological and medical indications including Skin Rejuvenation, Acne, Pigmentation,
Redness, Psoriasis, Wound Healing and Musculoskeletal Pain.
The Flex MD system is intended for use by non-trained public at home and trained skin
professionals via our training materials.
2. Important Safety Notice
Reading this User Guide is mandatory before attempting to operate
the Dermalux® Flex MD system. It is important to follow the operating
instructions and safety recommendations at all times. Any use of the
Dermalux® Flex MD system other than outlined in this User Guide is
considered to be improper and may cause damage which is not covered
under the warranty or could lead to an adverse response.

Aesthetic Technology Limited
6 7
PL-027 (Rev 7) PL-027 (Rev 7)
The Dermalux® Flex MD is a Medical Device for use on a treatment bed with a floor
standing option also available. The device emits specific wavelengths of low level, narrow
band light for the treatment of certain cosmetic, dermatological and medical indications.
The wavelengths used in the Dermalux® Flex MD system are Blue 415nm, Red 633nm and
Near Infrared 830nm.
The system enables treatment of the face and the body via a flexible LED Array. The Array
can be used in the Base for facial treatments or it can be removed from the Base to treat
larger body areas by laying the device directly over the treatment area.
The system consists of a Base unit, power supply unit (100-240Vac, 50/60Hz), capacitive
touch Controller and flexible Array that contains Light Emitting Diodes (LEDs).
The power supply is designed to power the internal electronics of the system and the Light
Emitting Diodes. The power supply is connected to a suitable mains outlet via a mains
outlet and wall plug. The device is switched ON/OFF by a button located on the Controller.
The Dermalux® Flex MD system is operated by a capacitive touch Controller, which allows
7 treatment protocols to be selected via various wavelength combinations.
The light is generated by Light Emitting Diodes (LED’s) which are contained within the
flexible LED Array. The LED Array can be placed into the Base, to secure the appropriate
position to administer a facial treatment. For use on the body, the LED Array is removed
from the Base unit and placed directly onto the body area.
Safety goggles are supplied for patient comfort and safety.
The equipment is not used to make measurements of any sort, or to draw any conclusions
regarding the indication to treat. The equipment does not require checks on the light output
as the LEDs do not dim with age to any practical extent.
4. Device Description 5. Device Components
• Flex MD Base Unit
• Flexible LED Array
• Flex MD Controller
• RJ11 Cable (2m)
• Power Supply (with 1.5m lead)
• Client Goggles
• Pillow
• Travel Carry Bag
• User Guide
• Protocol Chart
Figure 1: Flex MD Components.
Aesthetic Technology Limited
8 9
PL-027 (Rev 7) PL-027 (Rev 7)
1. Unpack and assemble the device as per PL-028 Assembly Guide found in Section
19 of the User Guide.
2. Once the Flex MD is unpacked, assembled and connected to a mains
socket, switch on the mains switch, then press the power button on the
Controller (Power button will illuminate in green).
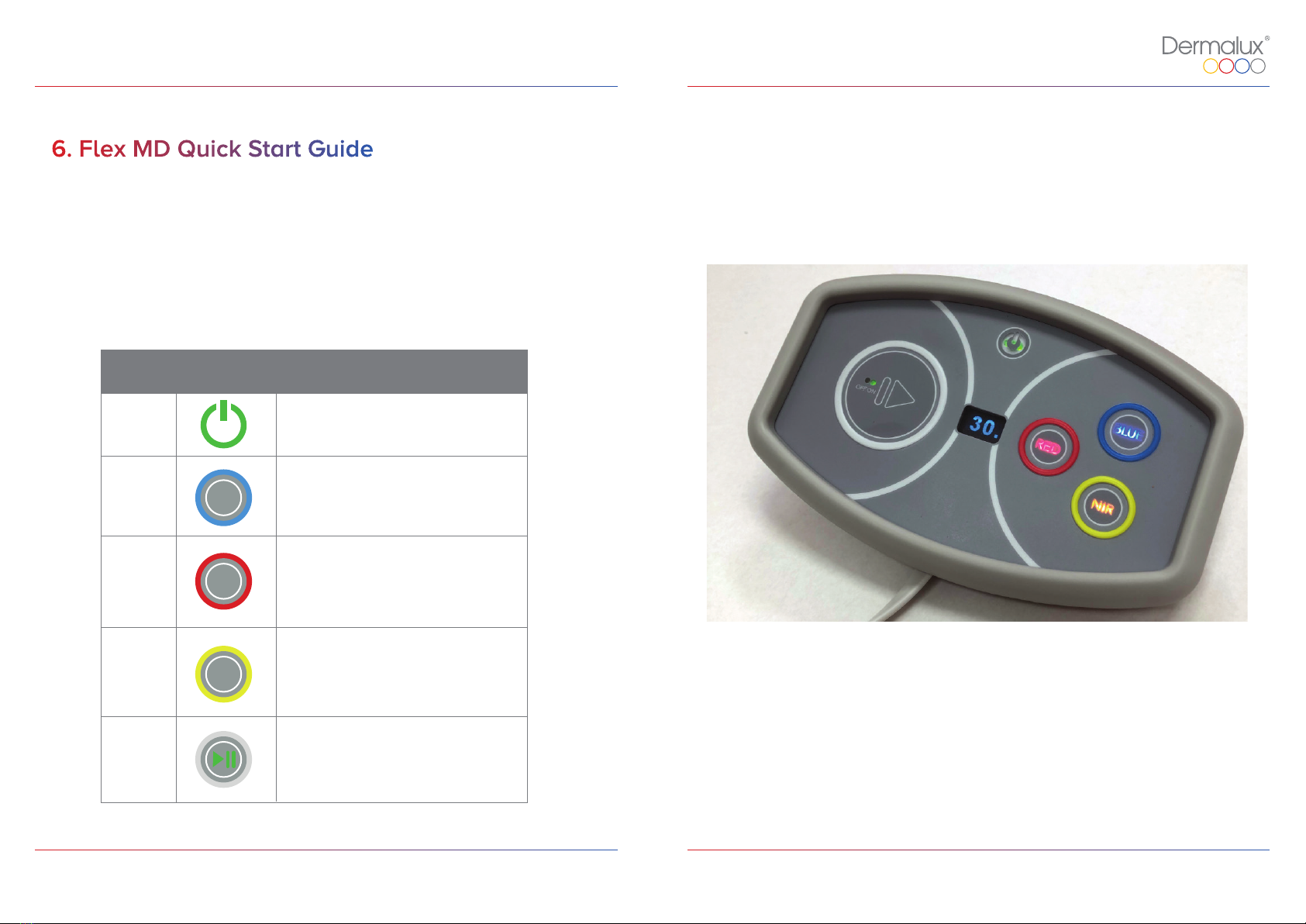
6. Flex MD Quick Start Guide
Name Icon Description
Power
Blue
Red
Turns device on and off.
Activates Blue LED’s for treatment.
Activates Red LED’s for treatment.
NIR Activates Near Infra-Red LED’s
for treatment.
Control Buttons
BLUE
RED
NIR
Play Initiates 30 minute treatment with
pre-selected LED wavelengths.
Table 1: Flex MD Control Buttons.
3. Upon activation, the timer screen will display the Dermalux® logo, followed by the
Flex MD logo.
Select the required combination of wavelengths by pressing the buttons on the right
side of the Controller. See Flex MD Treatment Protocol Chart (PL-025)
4. When the required wavelengths have been selected, press the PLAY button.
The PLAY button will then change from red to green to indicate that the treatment
has started. If the treatment is paused it will revert to a red light until resumed.
Figure 2: Flex MD Controller.

Aesthetic Technology Limited
10 11
PL-027 (Rev 7) PL-027 (Rev 7)
LED Phototherapy is well evidenced for its regenerative and anti-inflammatory benefits
without creating trauma, making it safe and suitable for all skin types. Due to the
non-invasive nature and high safety profile of the treatment, the scope for use extends
across a wide range of applications.
The Dermalux® Flex MD system delivers three clinically proven wavelengths via 7
treatment options selected through the Controller. These wavelengths are 415nm,
633nm and 830nm.
7. Indications for Use
• Skin Rejuvenation
• Complexion
• Dry Skin
• Pigmentation: Photo-damage
• Pigmentation
• Acne: Mild to Moderate
• Acne: Moderate to Severe
• Redness: Vascular
• Redness: Skin Tone
• Sensitive Skin
• Sensitive Skin: Problem conditions
• Psoriasis
• Wound Healing
• Pain
Refer to Flex MD Treatment Protocol manual (PL-038) for a detailed explanation
of protocols.
Indications for use include but are not limited to the following:
8. Precautions and Contraindications
The Dermalux® LED Phototherapy treatment is safe and well tolerated, however
there are certain circumstances and medical conditions in which treatment may
prove to be unsuitable.
Prior to treatment, we strongly recommend that all clients complete and sign the
Dermalux® Consent Form (PL-012, available at learn.dermaluxled.com) and disclose
any medications or medical conditions which may contraindicate the treatment at
the current time (available in our online materials). Failure to do so may increase
the risk of an unwanted or adverse reaction. For more information about Adverse
Reactions please refer to Section 14.
Aesthetic Technology Limited
12 13
PL-027 (Rev 7) PL-027 (Rev 7)
Indications for use include but are not limited to the following:
• Epilepsy or Seizures triggered by light
• Metabolic disorders such as Porphyria
• Autoimmune disorders such as Systemic Lupus Erythematosus (SLE)
• Photosensitive disorders such as Photosensitive Eczema
• Active Cancers
• Use of Photosensitive Medications
9. Contraindications 10. Warning Signs Associatedwith Photosensitivity
10.1 Other substances known to increase the risk of sensitivity
10.2 Recognising Photosensitivity
Certain medical conditions, medications and chemicals are known to induce
Photosensitivity. Photosensitivity is a common side effect of various medications.
These can include certain antibiotics, chemotherapy drugs, and diuretics.
St John’s Wort, anti-perspirant, antibacterial soaps, artificial sweeteners, naphthalene
(mothballs), petroleum products, brightening agents found in laundry detergent, and
cadmium sulphide (a chemical injected into the skin during tattooing).
Photosensitivity may also be caused by the use of certain perfume applied in the
treatment area.
Photosensitivity is a condition characterised by increased sensitivity to light, particularly
UV light. It refers to the development of a skin reaction as a result of the combined effects
of light and certain medications (oral and topical), hormonal changes, trauma or chemical
causes. General symptoms present as an erythema reaction similar to sunburn.
Exposure to either a chemical or the light alone is not sufficient to induce a photosensitive
reaction. When photo-activation of a chemical occurs, a phototoxic or photoallergic
reaction may arise.
For a list of Photosensitive Medications, please refer to the Dermalux Consultation Guide
(PL-011, available at learn.dermaluxled.com).
Aesthetic Technology Limited
14 15
PL-027 (Rev 7) PL-027 (Rev 7)
The Dermalux® Flex MD device has NOT been tested on pregnant women and therefore
the risk to the foetus or pregnant woman is unknown. Administration of the treatment during
pregnancy would strictly be at the users or practitioners discretion.
For further Information about Contraindications please refer to the Dermalux Consultation
Guide (PL-011, available at learn.dermaluxled.com).
11. Pregnancy
Clients who present with any of the following medical conditions may be treated with
caution and must be closely monitored.
Eye disease and conditions including Glaucoma, Cataracts, recent laser eye surgery
or Light Induced Migraines.
12. Precautions
There are no known allergic reactions to the materials used for the manufacture of this
device, should reaction occur please seek medical advice and report as per Section 14.
Immediately after Flex MD treatment:
• Skin may feel warm and may appear red, due to the increased blood circulation
in the treated area. Skin should return to normal within 1 to 2 hours.
• Temporary headaches immediately following the LED treatment (very rare).
• Headaches can be treated with an appropriate pain killer and rehydration.
13. Known Risks
Should injury occur as a result of using this device please seek medical advice and report
as per Section 14.
14. Adverse Event Reporting
In the unlikely event of a product fault or an adverse response to the Dermalux® Flex MD
treatment, please report the issue to Aesthetic Technology Ltd or your local Dermalux
representative as soon as possible. For all adverse reactions please complete form QOP-82-05-F1
following instructions provided with the form (available at learn.dermaluxled.com).
• A malfunction or deterioration in the characteristics or performance of the device.
- A malfunction or deterioration should be understood as a failure of the Dermalux®
device to perform in accordance with its intended purpose as specified in the User
guide.
• An adverse reaction or unanticipated side effect.
- An adverse reaction is an incident which causes, or has the potential to cause,
unexpected or unwanted effects involving the safety of persons receiving/or
having received a Dermalux® treatment or users of the Dermalux® LED system.
- An unanticipated side effect is one that exceeds the known side effects or is not
listed as a potential side effect in the Dermalux® TM User guide (anticipated side
effects)
• Use the correct section of the form to report;
- A malfunction or deterioration of the device
- An adverse reaction or unanticipated side effect
Examples of reportable events include:
Aesthetic Technology Limited
16 17
PL-027 (Rev 7) PL-027 (Rev 7)
WARNING! Use of accessories, transducers and cables other than those
specified or provided by the manufacturer of this equipment could result
in increased electromagnetic emissions or decreased electromagnetic
immunity of this equipment and result in improper operation.
Only use the power adapter and accessories supplied along with detachable parts with
the system or specified by the manufacturer and rated for country of use. Using any
other adapter may lead to an electrical fault or could result in increased electromagnetic
emissions or decreased electromagnetic immunity of this equipment and result in improper
operation
To fully isolate the device from the mains power, unplug from the mains outlet socket.
Ensure the mains outlet socket is always accessible.
The power lead supplied with the unit may present a trip hazard if installed incorrectly
or in the main footpath of operation. Always take caution when manoeuvring around the
product.
The power lead may present itself as a strangulation hazard to infants if not correctly installed.
Always plug the system into a direct mains outlet socket. Do not use an extension cable or
socket adaptor.
Do not use the system near water/liquids or where water/liquids could be spilt onto it.
Do not use the system if the LED Array has come into contact with water/liquids.
Do not plug, unplug or activate the system with wet hands.
Do not use the system in the presence of flammable liquids or gases.
Do not disassemble any parts from the system. Opening or removing covers may expose
the operator to dangerous voltages or other risks. Incorrect reassembly may lead to an
electric shock.
Do not operate or handle the device when powered if it is damaged in any way. Contact
Aesthetic Technology Ltd or your local Dermalux® representative for advice. If the LED
Array is damaged and powered it poses no risk to the user or operator due to low voltage
supply.
Do not attempt to modify the system in any way as this may result in an electric shock
when the system is used again. Unauthorised modification will also void the warranty.
Portable RF communications equipment (including peripherals such as antenna cables
and external antennas) should be used no closer than 30 cm (12 inches) to any part of the
Dermalux® Flex MD, including cables specified by the manufacturer. Failure to take notice
of this warning may result in degradation of the device.
WARNING! Avoid use around children and animals to prevent unnecessary risk.
The Dermalux® Flex MD system is an electrical device, which under certain circumstances
could present an electrical shock hazard to the user. Please follow the directions stated in
the manual and online training to assure maximum safety during operation.
15. General Safety Warnings

Aesthetic Technology Limited
18 19
PL-027 (Rev 7) PL-027 (Rev 7)
16. To Reduce the Risk of Damaging the System
• Do not place the system in a dusty or dirty environment.
• The system should be kept away from high temperature sources including direct
sunlight.
• When storing the system, place the LED Array in a flat position.
• The system may be cleaned by following the cleaning instructions in Section 20 of
this User Guide.
• Do not use any other cleaning products other than those stated in this User Guide.
Use of other cleaning products may damage the system.
• When not in use, it is advised to keep the system switched off.
• Avoid contact with children and animals.
WARNING!
17. Eye Safety
CAUTION! Protective eyewear should be worn by the user when the device is in use.
Do not stare at operating LED Array. May be harmful to the eyes!
Take note of the Warning label found on the inside of the Flex MD,
example listed below.
WARNING!
18. Receiving the Dermalux® Flex MD
Inspect the system for any visual damage that may have been caused during transit.
Check warranty labels are intact at time of receiving the device.
If any of the individual components are missing or damaged listed in Section 5 of this
document, please contact Aesthetic Technology Ltd or your local Dermalux® representative
within 24 hours of receiving the system.
Refer to Section 19 for a step by step assembly guide.
For reference the Dermalux® Flex MD system consists of:
• Flex MD Base Unit
• Flexible LED Array
• Flex MD Controller
• RJ11 Cable (2m)
• Power Supply (with 1.5m lead)
• Patient Goggles
• Pillow
• Travel Carry Bag
• User Guide
• Protocol Chart
Figure 3: Flex MD Components.

Assembled
LED Array
Pillow
Base
Goggles
User Guide
Controller
RJ11
Cable
Power Supply
Bag
Figure 4: Flex In Packaging.
Aesthetic Technology Limited
20 21
PL-027 (Rev 7) PL-027 (Rev 7)
19.1 Assembly Guide
Background
The Flex MD is delivered in one box (the product box) wrapped in another box (the
transport box). If being shipped overseas multiple Flex MD will be loaded into a carrier
and palletised. Care should be taken when unloading from the pallet. Make sure each box
remains in the correct orientation, follow markings on the box as a guide.
Warning
• Do not connect or activate the device until instructed.
• Do not use sharp objects to cut open the packaging as this may cause damage to
the items inside.
Packaging
All packaging has been specifically designed for transportation, please dispose of all
waste appropriately and only remove from the transportation packaging when necessary.
Labelling
All labels are positioned as required under the document guidelines of (PL-001)
Disclaimer
Aesthetic Technology Ltd takes no responsibility for any damaged products from
repackaged devices upon receipt of the shipped product.
19. Using the Device
Step 1: Remove product from packaging, as shown in Image 1.
Step 2: Place Base onto a flat surface and secure the LED Array into place,
as shown in Image 2.
Figure 5: Assembled Flex MD.
19.1.1. Unpacking and Set Up of Flex MD

Aesthetic Technology Limited
22 23
PL-027 (Rev 7) PL-027 (Rev 7)
Step 4: Connect Power Supply via port in LED Array and plug directly into
a mains socket. See Image 4.
Step 3: Connect Controller via RJ11 cable, as shown in Image 3.
Figure 6: Connecting Controller.
Figure 7: Connecting to Power.
The device does not require a warm up period. However, if stored above normal room
temperature conditions allow appropriate time to cool to normal operating conditions.
The maximum time to wait upon set up from a max storage temperature is 15 minutes.
There is no risk to the device, user or operator if using the device from the minimum
storage temperature of -25 Degree’s Celsius.
• Mount Flex MD LED Array into Base unit
• Connect the RJ11 cable to the Controller
• Connect the opposite end of the RJ11 connector to the Flex MD LED Array
• Connect the Power Supply to the Flex MD LED Array using the 5mm barrell jack
• When plugging in to the mains wall socket, ensure the socket is off before plugging in
• Now switch the wall socket on
• Press the power button on the Controller to activate
• Display will illuminate with the following, in order:
- Dermalux logo
- Flex MD logo
- 30 (minutes)
• When the Controller is functional, Select required wavelengths. On selection, colours
will illuminate
• To start the treatment, press Play. To pause the treatment, press Pause
• The Play / Pause LED will switch when Play / Pause is pressed.
Green indicates Play, Red indicates Pause
- All wavelength outputs are constant
- Time runs from 30 minutes only on countdown
- When the treatment has finished a “00” will display
19.2 Setup and First use

Aesthetic Technology Limited
24 25
PL-027 (Rev 7) PL-027 (Rev 7)
19.3 Startup & Shutdown
19.3.1 Startup
Once the Dermalux® Flex MD is connected to a mains socket, switch on using the power
button on the Controller (the button will light up in green). Refer to icon in Table 2 on page
25. Button Reference ID 1.
19.3.2 Shutdown
To initiate a shutdown, press the power button on the Controller. When not in use unplug
from the mains power socket. Refer to icon in Table 2 on page 25. Button Reference ID 1.
19.4 Selecting a Program
The following table identifies the universal icons that shall be used throughout the
Controller display software.
The Flex MD device does not require a cool down period
19.4.1 Icons
Name Icon Description Button
Reference ID
Power Turn device on and o 1
Blue Activates Blue LED’s for
treatment
2
Red Activates Red LED’s for
treatment 3
NIR Activates Near Infra-Red
LED’s for treatment 4
5Play
Initiates 30 minute
treatment with pre-selected
LED wavelengths.
*Note the key functions will be labelled throughout the document as their button reference number.
BLUE
RED
NIR
Table 2: Flex MD Control Buttons.

Aesthetic Technology Limited
26 27
PL-027 (Rev 7) PL-027 (Rev 7)
19.4.2 Detailed Software Walk Through
1. If the POWER button is pressed at any time the device will turn off and any treatment
will be cancelled.
2. If the PLAY button is pressed any treatment in progress will pause until resumed
(by pressing PLAY again) or cancelled (by pressing the POWER button).
3. If the BLUE, RED or NIR buttons are pressed at any time during a treatment,
the corresponding wavelengths will be selected or deselected.
Button/ ID Function Requirement
N/A Upon successful completion of initialisation, the POWER
button will illuminate in green.
2
In the HOME screen, if the BLUE button is pressed, the blue
LED’s will be selected for the treatment and the button will
illuminate Blue.
*To deselect blue LED’s press BLUE button again.
3
In the HOME screen, if the RED button is pressed, the red
LED’s will be selected for the treatment and the button will
illuminate Red.
*To deselect red LED’s press RED button again.
4
In the HOME screen, if the NIR button is pressed the near
infra-red LED’s will be selected for the treatment and the
button will illuminate Yellow.
*To deselect near infra-red LED’s press NIR button again.
5
In the HOME screen, if the PLAY button is pressed the
treatment will initiate with whichever LED wavelengths
were previously selected.
*If no wavelengths have been selected (BLUE, RED, NIR) the
PLAY button cannot be selected.
* Navigation: Home
Table 3: Flex MD Function
Overview.
Figure 8: Controller Overview, Home.

Aesthetic Technology Limited
28 29
PL-027 (Rev 7) PL-027 (Rev 7)
19.4.3 Red 633nm Treatment
* Navigation: Home - Red Treatment
Button/ ID Function Requirement
3Press the RED button to activate the red LED’s.
This is indicated by the button illuminating in red.
5In the RED TREATMENT screen, if the PLAY button is
pressed the treatment is in progress or Paused.
Figure 9: Controller Overview for Red Treatment.
19.4.4 Blue 415nm Treatment
Button/ ID Function Requirement
2Press the BLUE button to activate the blue LED’s.
This is indicated by the button illuminating in blue.
5In the BLUE TREATMENT screen, if the PLAY button is
pressed the treatment is in progress or paused.
* Navigation: Home - Blue Treatment
Figure 10: Controller Overview for Blue Treatment.

Aesthetic Technology Limited
30 31
PL-027 (Rev 7) PL-027 (Rev 7)
19.4.5 NIR 830nm Treatment
* Navigation: Home - NIR Treatment
Button/ ID Function Requirement
4Press the NIR button to activate near infra-red LED’s.
This is indicated by the button illuminating in yellow.
5In the NIR TREATMENT screen, if the PLAY button is
pressed the treatment is in progress or paused.
Figure 11: Controller Overview for NIR Treatment.
19.4.6 Blue & Red Treatment
Button/ ID Function Requirement
2Press the BLUE button to activate the blue LED’s.
This is indicated by the button illuminating in blue.
3Press the RED button to activate red LED’s.
This is indicated by the button illuminating in red.
* Navigation: Home - Blue & Red Treatment
5In the BLUE & RED TREATMENT screen, if the PLAY button
is pressed the treatment is in progress or paused.
Figure 12: Controller Overview for Blue & Red Treatment

Aesthetic Technology Limited
32 33
PL-027 (Rev 7) PL-027 (Rev 7)
19.4.7 Blue & NIR Treatment
* Navigation: Home - Blue & NIR Treatment
Button/ ID Function Requirement
2Press the BLUE button to activate blue LED’s.
This is indicated by the button illuminating in blue.
4Press the NIR button to activate near infra-red LED’s.
This is indicated by the button illuminating in yellow.
5In the BLUE & NIR TREATMENT screen, if the PLAY button
is pressed the treatment is in progress or paused.
Figure 13: Controller Overview for Blue & NIR Treatment.
19.4.8 Red & NIR Treatment
Button/ ID Function Requirement
3Press the RED button to activate red LED’s.
This is indicated by the button illuminating in red.
4Press the NIR button to activate near infra-red LED’s.
This is indicated by the button illuminating in yellow.
* Navigation: Home - Red & NIR Treatment
5In the RED & NIR TREATMENT screen, if the PLAY button is
pressed the treatment is in progress or paused.
Figure 14: Controller Overview for Red & NIR Treatment.
Aesthetic Technology Limited
34 35
PL-027 (Rev 7) PL-027 (Rev 7)
19.4.9 Tri-Wave Treatment
* Navigation: Home - Tri-Wave Treatment
Button/ ID Function Requirement
2Press the BLUE button to activate blue LED’s.
This is indicated by the button illuminating in blue.
3Press the RED button to activate red LED’s.
This is indicated by the button illuminating in red.
4Press the NIR button to activate near infra-red LED’s.
This is indicated by the button illuminating in yellow.
5In the TRIWAVE TREATMENT screen, if the PLAY button is
pressed the treatment is in progress or paused.
Figure 15: Controller Overview for Tri-Wave Treatment.
It is important that the Dermalux® Flex MD system is switched off and unplugged from the
mains power socket before cleaning.
The entire surface of the system including the LED Array, Base unit and Controller may be
cleaned with an anionic detergent towelette or surface wipe. Excess moisture from the wipe
may be removed by using a clean dry microfibre cloth.
Please refrain from using any other cleansing products as these may damage the plastics
and/or electrical components.
If the Dermalux® Flex MD system is used directly on the body, the device must be
thoroughly cleaned before use on another client.
The device will require no manufacturing maintenance or service on an annual basis. The
customer is expected to carry out routine maintenance such as cleaning and general health
inspections of the device on a quarterly basis. This will include visual inspection of exposed
wires, material integrity and any signs of damage in the plastic componentry.
No maintenance will be performed on the Dermalux® Flex MD system by the manufacturer.
Any faults found with the system within the warranty period should be reported to Aesthetic
Technology Ltd or the local Dermalux Representative and a replacement device will be
issued if required.
The Dermalux® Flex MD system contains no serviceable parts.
Calibration of the system is not required.
There is no annual maintenance service with this product.
20. Cleaning Instructions
21. Maintenance
22.ServicingRequirements

Aesthetic Technology Limited
36 37
PL-027 (Rev 7) PL-027 (Rev 7)
23. Storage & Transit Conditions
24. Operating Conditions
When not in use store the Dermalux® Flex MD system at room temperature in dry/low
humidity conditions.
Environmental conditions for transport and storage: -25 to +70 degrees Celsius and up to
90% humidity (normal range for room temperature conditions). Atmospheric pressure range
of 50 kPa to 106 kPa
Store away from direct heat and sunlight.
The Dermalux® Flex MD system should be operated at room temperature in dry/low
humidity conditions. 5-40 degrees Celsius and 15-90% humidity (normal range for room
temperature conditions). Atmospheric pressure range of 70 kPa to 106 kPa
Keep away from direct heat and sunlight.
The duty cycle for this product is continuous.
The product lifetime of this device is 2 years. Aesthetic Technology Ltd provides a 2 year
warranty to match the intended lifetime of the device.
Do NOT dispose of any part of the Dermalux® Flex MD LED system at a domestic waste
facility. Seek waste disposal advice from your local council or consult Aesthetic Technology
Ltd for guidance.
For protection of the environment, please package and return this unit to Aesthetic
Technology Ltd or their representative at the end of its working life.
On 27th January 2003, the European Parliament and the Council of the European Union
authorized Directive 2002/96/EC on waste electrical and electronic equipment (WEEE). This
directive encourages and sets specific criteria for the collection, handling and recycling of
electric and electronic waste.
25. Disposal

Aesthetic Technology Limited
38 39
PL-027 (Rev 7) PL-027 (Rev 7)
26. Technical Specification
• Blue (415nm)
- Maximum optical power is 10 j/cm2
- Maximum operating time per treatment is 30 minutes
• Red (633nm)
- Maximum optical power is 20 j/cm2
- Maximum operating time per treatment is 30 minutes
• NIR (830nm)
- Maximum optical power is 10 j/cm2
- Maximum operating time per treatment is 30 minutes
• Maximum variation of 10% optically
• Ocular hazard distance (EN 60601-2-57): 0.025m when in use with the Base unit,
0.0m when directly placed on the body
• EN 62471 Risk Group 2
• Mains supply: 100 -240 Vac, 50 / 60 Hz, 1A.
• PSU output, 30Vdc, 1.2A 36W
• Tailor to clients with 7 treatment options.
• Weight: 2.5kg
• Footprint (in Base Unit) W520 x L335 x H300 mm
• Footprint (Array in flat position): W280 x L790 x H30 mm
• Duty Cycle: Continuous
• IP Rating : IP20
26.1 Electromagnetic Guidance
Emissions Test Compliance
RF conducted and radiated emissions CISPR 11 Group 1, Class B
Harmonic emissions IEC 61000-3-2 Class A
Voltage fluctuations/ flicker emissions IEC 61000-3-3 Complies
Immunity tests Immunity Test Level
Electrostatic Discharge (ESD)
IEC 61000-4-2
± 8 kV contact
± 2 kV, ± 4 kV, ± 8 kV, ± 15 kV air
Radiated RF EM fields
IEC 61000-4-3
10 V/m
80 MHz – 2,7 GHz
80 % AM at 1 kHz
Proximity fields from RF
wireless communications equipment
IEC 61000-4-3
380 – 390 MHz 27 V/m
430 – 470 MHz 28 V/m
704 – 787 MHz 9 V/m
800 – 960 MHz 28 V/m
1.7 – 1.99 GHz 28 V/m
2.4 – 2.57 GHz 28 V/m
5.1 – 5.8 GHz 27 V/m
Electrical fast transients/burst
IEC 61000-4-4
± 2 kV
100 kHz repetition frequency
Surges
IEC 61000-4-5 Line to line: ± 0,5 kV, ± 1 kV
Conducted disturbances induced by RF fields
IEC 61000-4-6
3 V/m
0,15 MHz – 80 MHz
6 V/m in the ISM and Amateur Radio bands between
0,15 MHz and 80 MHz
80 % AM at 1 kHz
Voltage dips and interruptions
IEC 61000-4-11
0 % UT; 0,5 cycle
At 0°, 45°, 90°, 135°, 180°, 225°, 270° and 315°
0 % UT; 1 cycle at 0°
70 % UT; 30 cycles at 0°
0 % UT; 300 cycles
UT is the AC mains voltage prior to application of
the test level.
Table 11: Electromagnetic Technical Information.
Table of contents
Popular Personal Care Product manuals by other brands

Vitalmaxx
Vitalmaxx BJ-1357 instruction manual

Direct Healthcare Services
Direct Healthcare Services Dyna-Form Air Pro-Plus user manual

Light Mandalas
Light Mandalas Mandala Resonator user manual

Silk'n
Silk'n RM249-DL user manual
Antar
Antar AT92068 user manual

Novo Nordisk
Novo Nordisk Victoza Pen Getting started with











