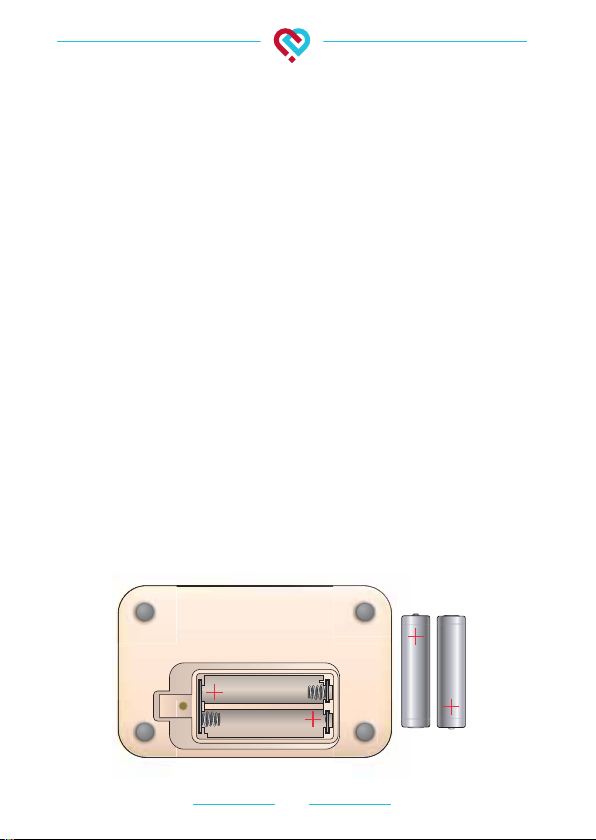
8
8. Directions for use of the device
Point of eect.
The device has a mild, harmless eect on the body, and can
be used for both adults and children with no age restrictions.
Therapy is carried out by a course of treatment consisting of
individual sessions.
The therapeutic eect is achieved by the impact of the elec-
tromagnetic eld on the human body.
Due to the high penetrative capability of the electromagnet-
ic eld, it is not necessary to remove clothing. It is necessary to
place the device next to the aected organ to obtain the most
pronounced therapeutic eect.
Session - this is a one-time therapeutic eect from a specic
program. You can undergo several sessions spread throughout
the day.
The most pronounced therapeutic eect results from a
course of treatment. In this case, a course of treatment is
6-day’s exposure followed by a one day break in conducting
sessions. For advanced diseases, treatment may require up to
5-6 courses.
It should be noted that during therapy, the underlying dis-
ease may be aggravated, which may be accompanied by gen-
eral tiredness, a temperature, weakness, etc., which is associ-
ated with the elimination of the infectious pathogen. In this
case, the interval between sessions should be increased to feel
better. This is extremely important and compulsory, and must
not be ignored.