Diagnosis Diagnostic NANO User manual
NANO
NEBULIZER
PISTON COMPRESSOR
INSTRUKTION MANUAL
Diagnosis S.A.
ul. Gen. W. Andersa 38A
15-113 Białystok, Poland
www.diagnosis.pl
0197
7015
EN
DEAR CUSTOMER
Thank you for buying the Diagnostic NANO nebulizer intended for inhalation. Inhalation therapy is an
efficient and safe method of treatment of respiratory diseases. The treatment should be started after
consultation with your doctor. Before the first use of the nebulizer, carefully read the instructions for use.
1. INTRODUCTION
Diagnostic NANO is a compact medical device intended for intermittent use (30 min operation, 30 min
break). Enables the medical preparation to immediately and directly reach bronchial tubes and lungs as
an inhalant. If proper precautionary measures are taken, the nebulizer will ensure appropriate treatment
quality for a number of years.
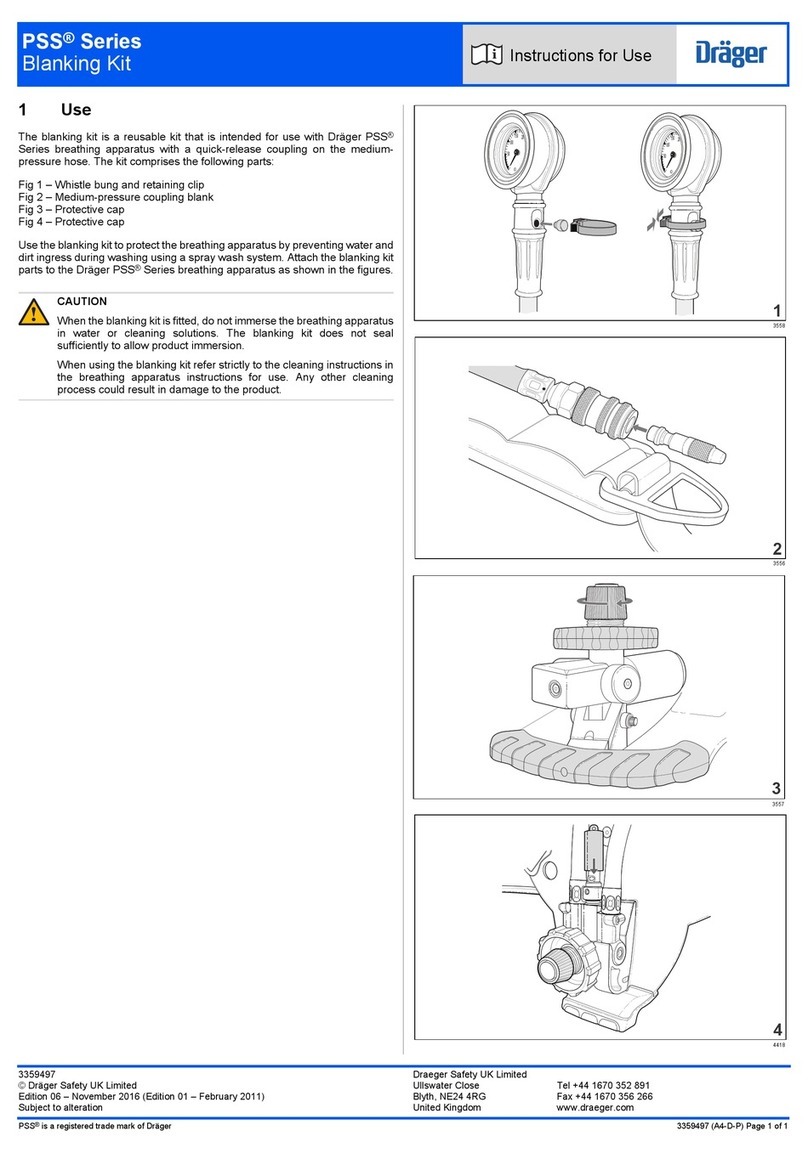
2. OPERATING DESIGN
IThe Diagnostic NANO nebulizer was designed for the treatment of asthma,
allergies and other respiratory diseases. The device produces a stream of air
led through the air tube to the nebulizer. After the introduction of air into the
nebulizer, the medical preparation will be atomized, creating an easily-inhaled
mist. The main flow of air into and out of the compressor is presented in
Figure 1.
We advise you to read this user manual in order to familiarize yourself with
the characteristics of the product. The product should not be used for other
than the intended purpose.
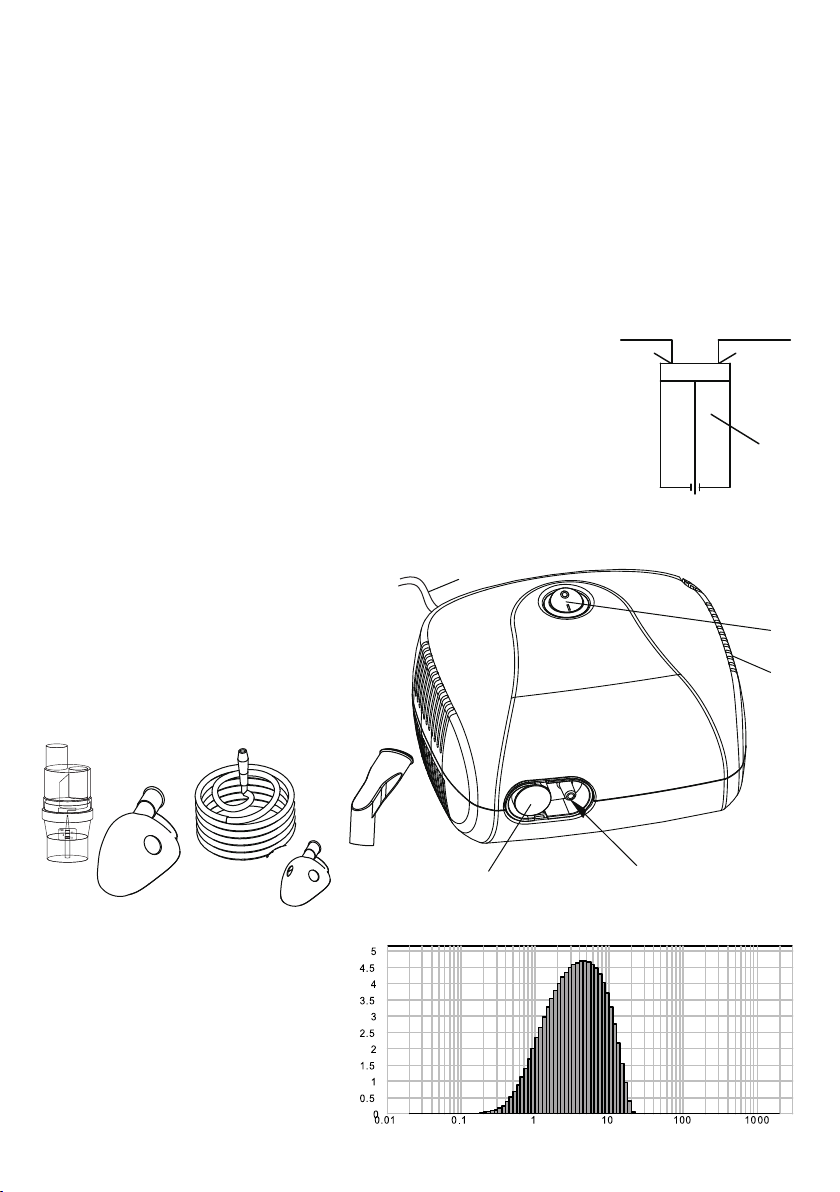
3. PRODUCT FEATURES
Before using the device, make sure
that the following elements are included:
1 x main unit (compressor)
1 x nebulizer (spray nozzle)
1x air tube
1x mask for adulst, mask for children
1x mouthpiece
5x air filter
air
filter
inlet outlet
compressor
air
outlet
Fig.1
Air tube
Mouthpiece
Mask
for adults
Mask
for children
Nebulizer
(spray nozzle)
MAIN UNIT
(COMPRESSOR)
1
On/off
switch
Vents
Power
cord
Air tube
socket
Filter
and filter cover
Fig.2
SPECIFICATION OF PARTICLES` SIZE
ATOMIZED BY THE NEBULIZER
Data may vary depending on medicine
used. Measurements done by using
MALVERN technology and physiological
salt solution. Data provided may not be
applicable to suspended solids or to
high viscosity medicine therefore, in
case of using medicine different than
physiological salt solution please
contact the manufacturer of the
medicine used for inhalation
Łączna ilość rozpylanych cząsteczek w %
Wielkość rozpylanych cząsteczek (µm)
DiSTRIBUTION oF pARTICLES sIZE iN cOMLIANCE wITH EN 13544-1

AEROSOL THERAPY SYSTEM FOR HOME USE
IMPORTANT WARNINGS: failure to comply with the warnings and the contents of this user manual
may expose the user to such risks as: deterioration of health, burns, electric shock, recurrent
infections, death, fire, environmental pollution. The use of any electrical appliance requires
observance of the following fundamental principles:
BEFORE USING THE APPLIANCE CAREFULLY READ
THIS USER MANUAL
Failure to follow the user manual relating to the operation
of the device may cause malfunction.
Follow the electrical specifications stated on the device;
Concerning the issues related to the medication type,
dosage and usage schedule, the advice of a doctor must
be followed. The intensity of use of the device must
always be consulted with a physician.
The inhalation of any substances should be carried out
with permission of the doctor who decides on doses and
usage;
Lack of electricity supply, sudden failure or other adverse
conditions may cause device malfunctions; for that
reason, it is recommended to equip oneself
with a device or medication (in accordance with the
doctor's recommendations) that could be used
interchangeably;
Use the device only for its intended purpose (aerosol
inhalation) and in the manner described in this manual;
Do not allow the device to be used by children or persons
with disabilities without supervision;
in such cases, special care must be taken during therapy;
Do not use the device if the plug or power cord are
damaged.
Never leave the device, its accessories, or packaging
within the reach of children, unauthorized persons, pet
animals or insects.
If necessary, use adapters or extension cords compliant
with the applicable safety standards, paying attention not
to exceed the limit of current load and reducing the
maximum power indicated on the adapter;
Do not use the device with wet or damp hands, do not
use it while showering;
Never allow the device to be immersed in water or other
liquids; do not use the device if it has been accidentally
soaked;
The device and the power cable should be located away
from heat sources;
The device is equipped with liquid ingress protection;
Do not use the device near flammable objects or
explosives;
Do not use the device in an environment where sprays
have recently been used; ventilate the room before
starting therapy;
Do not block or clog vents on the device, do not place it
on a soft surface such as a bed or couch;
Do not insert any objects into the vents;
Do not use the device if it emits abnormal sounds;
In order to prevent deterioration of the device's
functionality, use only original accessories;
Accessories should only be used by one person. In the
event that the nebulizer is used by other family
members, we recommend cleaning the them before
each use or purchase additional accessories intended for
individual use only.
Do not pull the power cord or the appliance itself in order
to remove the plug from the electrical outlet.
After use, always unplug the power plug.
Do not expose the device to atmospheric conditions;
store it at room temperature.
Do not put the air tube or the power cord on the neck
The device contains small parts that may be dangerous if
swallowed.
Do not use the device in combination with accessories
other than those described in the user manual.
Do not use the device if it was stored in a polluted
environment.
RESPONSIBILITY
In terms of safety, efficiency and reliability, the
responsibility lies with the manufacturer only if:
assembly, calibration, repairs or modifications are carried
out by authorized persons;
electrical installation complies with the applicable
standards;
the user manual has been complied with. The
manufacturer shall not be liable for the improper,
incorrect or unreasonable use of the appliance.
FILTER REPLACEMENT
The filter should be replaced every 3 months or every 300
uses. To replace the air filter, follow these steps:
1. Remove the filter cover.
2. Replace the filter.
3. Reinstall the filter cover.
NOTE: If the filter becomes wet, it should be completely
dried before use
FUSE
There is a fuse installed inside the device, which must be
replaced in the event of a failure . The replacement
operation, as well as other possible repairs, should be
carried out by the manufacturer or its authorized personnel
ELECTROMAGNETIC COMPATIBILITY
The device complies with current standards regarding
electromagnetic compatibility and is suitable for use in all
buildings, including residential buildings. The radio
frequency emissions level of the device is very low and
does not cause interference with devices that are located
nearby. However, it is recommended that you do not
position it on or near other devices. If interferences with
other electrical equipment occur, the device should be
moved away or plugged into another electrical outlet.
Radio communication equipment may affect the operation
of the device. Keep it at a distance of at least 3 m from the
device.
2
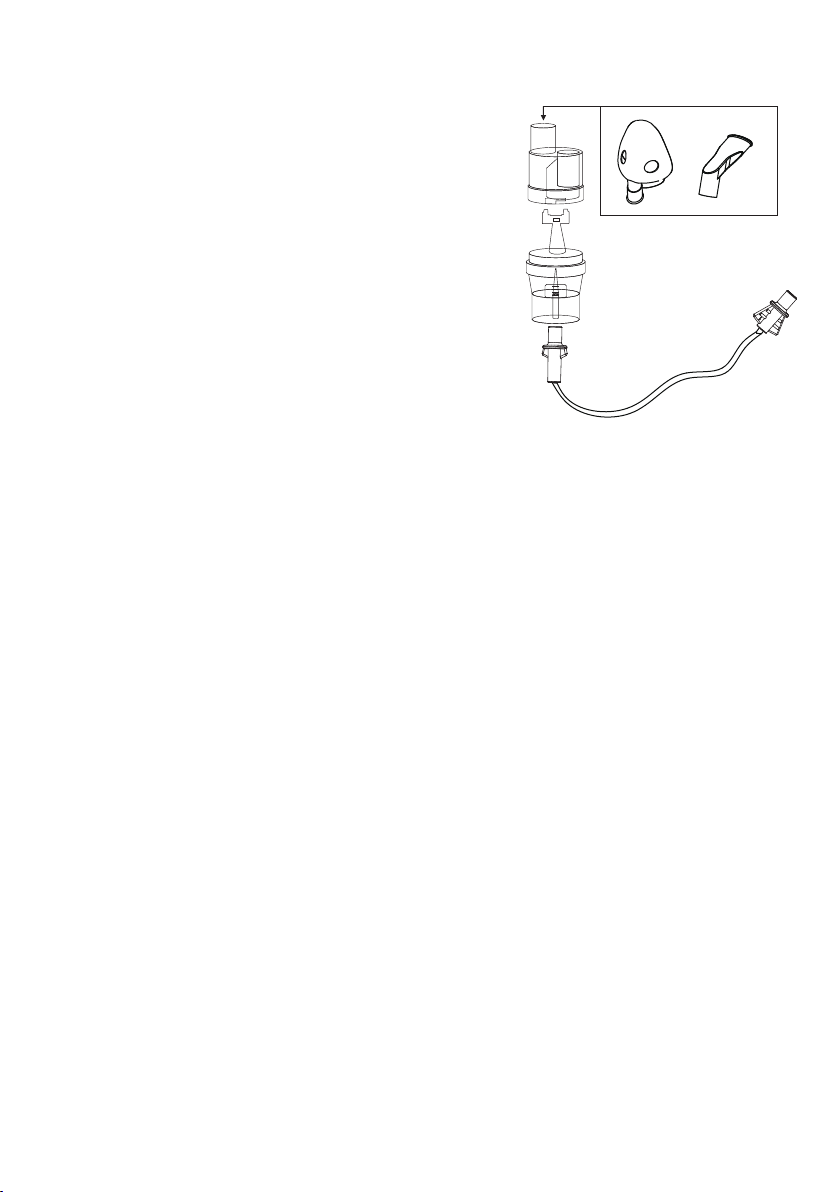
4. PREPARING FOR NEBULIZATION
To pour the medication into the nebulizer,
perform the following steps:
1. Open the medication cup.
2. Fill the medication cup in accordance with
the doctor's recommendations. Make sure
that the medication does not exceed the
volume of 8 ml.
3. Close the medication cup.
4. Attach the mouthpiece or mask.
NOTE: The mouthpiece is designed for oral
inhalation.
NOTE: Remember to use the baffle in the nebulizer
(in the medication cup). It is necessary for the atomizer
to work properly
5. HOW TO USE THE DEVICE
To start the nebulizer, perform the following steps:
Connect the plug of the compressor to the mains, but do not connect the nebulizer.
Make sure that the air filter is clean and located in the device.
Connect the nebulizer using air tube with the main unit (compressor).
Turn on the power.
Start inhalation in accordance with the doctor's recommendations.
Nebulizer operation time: 30 min operation / 30 min break. After 30 minutes of operation, the
device should be turned off in order to prevent compressor overheating.
Inhalation is completed when there is no more medicament in the cup.
After the end of the inhalation, turn off the unit before disconnecting the air tube
6. CLEANING
All activities associated with cleaning of the device should be carried out with the plug removed the from
the electrical outlet. In order to avoid incorrect operation of the device and inhaling undesirable
substances, clean the appliance and the accessories immediately after finishing therapy. Cleaning the
appliance should be carried out using a cloth slightly moistened with alcohol. After cleaning the
equipment, before using it, wait for the alcohol to evaporate completely. Do not use other liquids or
cleaning agents. Do not use cloths that are too wet, since the contact of liquids with electrical devices
may lead to operational failure, irreversible damage, as well as can be dangerous to your health.
7. CLEANING THE ACCESSORIES
1. Before the first use and after using the device each time, thoroughly clean the accessories
(except the power cord) with lukewarm water and dry with a cloth. They should be
subsequently placed in a clean place. During cleaning, make sure to have removed any
residues of substances intended for inhalation and then thoroughly dry the accessories. In any
event, do not use cleaning and disinfecting agents that may be toxic in contact with the skin
or mucous membranes, when swallowed or through inhalation. Any moisture remaining in the
air tubes should removed by starting the unit for a few minutes without the nebulizer
connected. If the air tube is dirty, it must be replaced.
max
6
4
2
ustnik
maska
pokrywa
pojemnika
na lek
dysza
pojemnik
na lek
przewód
powietrzny
Rys.3
BUDOWA nEBULIZATORA
(rOZPYLACZA)
3

2. In order for the nebulizer to work properly, after each use do the following:
After you disconnect the air tube remove the mouthpiece or mask.
Open the cap of the medication cup and empty it. Wash your nebulizer under running water or
leave it in warm water for 15 minutes. To achieve a better cleaning effect, add some vinegar
to water (a mixture of 1/3rd white vinegar, 2/3rds water).
NOTE: Do not boil the accessories as this may result in damage.
Do not put nebulizer accessories into the dishwasher.
Prior to an extended storage period, the accessories should be completely dried .
8. SPARE ACCESSORIES
To ensure effective inhalation, proper operation and safety, use only original accessories available for
purchase. All the accessories are suitable for direct contact with the skin. They do not contain
phthalates. In case of doubt, please contact Diagnosis. Free infoline 800 70 30 11.
9. PERIODIC INSPECTIONS
To maintain high effectiveness of the device, it is recommended to replace the medication cup every
12 months (every 6 months if the cup is used 3 times a day). A damaged medication cup should be
replaced immediately.
4
NOTE! Specifications may change without prior notice.
10. TECHNICAL SPECIFICATION
Power
Supply Power
Nebulizer flow
Compressor flow
Pojemność pojemnika na lek
Particle size
MMAD
Nebulization efficiency
Deposit quantity
Working time
Noise level
Operating conditions
Storage conditions
Dimensions (L x W x H)
Device weight
Accessories
230V AC, 50Hz
below 65W
6 l
8-10 l
2-8 ml
0,5 do 6 microns
2,44 microns
0,4 ml/min
0,15 ml
30 min operation / 30 min break
below 55 dBA
10°C do 40°C (50°F do 104°F)
10% to 95% relative humidity
-20°C do 70°C (-4°F do 158°F)
10% to 95% relative humidity
167 x 142 x 93 mm
1,51 kg
Nebulizer, mask (large), mask (small), 5 x air filter,
air tube 150 cm, mouthpiece

11. PrOBLEM sOLVING.
Problem
Nebulizer does
not start
Too noisy
Water drops are
formed in the air
tube.
Probable cause
The main switch of the
compressor is switched off.
No air filter in the device.
Nebulizer has not been
cleaned after previous use.
Air tube is folded.
Filter is clogged.
No medication.
Too much medication added.
Air tube has not been dried.
Solution
Turn on the device.
Install filter in the unit.
Clean the device.
Remove any bends or knots of the air tube.
Replace the filterr.
Add the appropriate quantity of drug prescribed
by the doctor to the medication cup.
Adjust the amount of the drug in the cup to the
proper level, connect the air tube to the
compressor and turn on the device.
The remaining moisture in the air tubing can be
removed by starting the unit for a few minutes
without connecting the nebulizer. If the air tube
is dirty, it must be replaced.
NOTE: If you experience any other problems with the device, contact Diagnosis.
Free hotline: 800 70 30 11
12. EXPLANATION OF SYMBOLS USED:
Portable wireless communication equipment may affect MEDICAL ELECTRICAL DEVICES
Working in the vicinity (within a distance of 2.8 m) of a cellular phone may cause instability of the stimulator
.
WARING!
WARING! Specifications required by the abovementioned standard are available at
http://www.diagnosis.pl/norma-en-60601-1-2.html
5
The worn out product should be taken to a waste collection facility. Contains components that
are dangerous for the environment. The correct disposal of the device allows to preserve
valuable resources and avoid negative effects on health and the environment, which may be
threatened by inappropriate handling of waste.
Important
warnings
Prąd
zmienny Manufacturer Produciton
year
Date of the
last review
Serial
number
Catalog
Number
Fuse
Rev. SN
Isolation
Class II
Urządzenie
Typ BF
IP21
Read
the user
Protect
before
moisture
Degree
of
protection
Rev. 2016.09.13
przygotowana na podstawie polskiej wersji językowej z dnia 2015.09.04
an

NOTES

1. Diagnosis S.A. grants a warranty:
2 years for DIAGNOSTIC NANOnebulizer (excluding accessories)
12 months for nebulizer accessories
Hardware defects revealed during the warranty period will be rectified free of charge within 21
days.
The term runs from the date of delivery of the equipment to the service center.
2. The purchaser shall be entitled to replace the equipment for a new one, free of defects,
when:
the repair has not been made within the time limit set in item 1
an authorized service center found an irreparable manufacturing defect
during the warranty period, 4 repairs were effected, and the equipment still shows defects that
prevent its use in accordance with its intended purpose.
The concept of repair shall not include operations related to equipment check and cleaning.
3. The warranty shall not cover: batteries, products with illegible or damaged serial number,
damage due to the operation and storage inconsistent with the user manual, ingress of liquids
or foreign bodies, overvoltage of mains, repairs by unauthorized persons and random events.
4. Faulty equipment should be delivered by the buyer to the address of the main service center
or one of the Authorized Service Centers (listed in the appendix).
5. The warranty for the sold consumer goods shall not exclude, restrict, or suspend the powers
of the buyer resulting from non-conformity of the goods with the contract .
6. The only basis for the warranty rights shall be the warranty card with the date of sale, stamp
and signature of the salesperson. If the card is not completed, filled in wrongly, with traces
of corrections and entries made by unauthorized persons, illegible as a result of damage - it
shall be invalid.
WARRANTY TERMS
WARRANTY CARD
Diagnosis S.A.
ul. Gen. W. Andersa 38A, 15-113 Białystok, Poland
www.diagnosis.pl
DEVICE NAME . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . MODEL . . . . . . . . . . . . . . . . . . . . . . . .
SERIAL NUMBER ..................................DATE OF SALE . . . . . . . . . . . . . . . . . .
store stamp and signature of salesperson
EN
Table of contents