Dittmann ASG 341 User manual

ASG 341
1
•
•
•
•
•
•
•
0123
Version GB 3, 2013-04
Snore stopper with electric stimulation current
1 channel system with 2 adhesive electrodes on the
inside of the device
Flexible wristband, individually adjustable up to 22 cm
wrist size
Noise sensitivity from 65 dB (decibel) if background
noises in the room are below 55 dB (decibel)
Contains: 1 snore stopper with adhesive electrodes, 2
spare adhesive electrodes, 3 alcoholic cleaning wipes,
1 instruction manual
1 x 1.5 V AAA battery
Warranty: 24 months
INSTRUCTION MANUAL
Snore Stopper ASG 341 GB
ASG 341
1
•
•
•
•
•
•
•
0123
Snore Stopper ASG 341 GB

1.0
2.0
2.1
2.2
3.0
3.1
3.3
3.4
3.5
3.6
3.7
3.8
4.0
4.1
4.2
4.3
5.0
6.0
7.0
8.0
9.0
10.0
10.0
10.0
11.0
4
4
4
4
5
5
5
6
6
6
7
7
8
8
8
8
9
9
9
10
11
12
12
13
14
2
Technical problems, troubleshooting
Application of the snore stopper, step 9 to 14
Application of the snore stopper, step 1 to 8
Application of the snore stopper
Designation and function of the snore stopper
Changing batteries and information about batteries
Disposal of the snore stopper
Information about noise sources, noise scale up to 70 dB (decibel)
Scope of delivery/contents of packaging
Cleaning and care of the snore stopper
Storage/maintenance of the snore stopper
Where the adhesive electrodes must not be attached
Where the adhesive electrodes must be attached
Using the snore stopper
Usage by children and adolescents
Application for which the snore stopper is not suited
Application for which the snore stopper is suited
Usage/environment for which the snore stopper is not suited
Usage/environment for which the snore stopper is suited
General safety instructions
Safety instructions
Information about snoring
How does a snore stopper work?
Basic information
Definition of symbols
No. Topic Page
TABLE OF CONTENTS
GB

12.0
13.0
14.0
19
20
3
Dear purchaser,
Congratulations on your purchase of your new snore stopper ASG 341
and thank you for your trust. To ensure optimal functionality and
operation of your snore stopper, please first read the instruction
manual before using the device. This guarantees a long useful life of
this product.
Terms of warranty
Technical specification, symbols, pictograms
15 - 18
Information regarding electromagnetic immunity
No. Topic Page
TABLE OF CONTENTS GB

4
Snoring is a rattling sound that is caused by a blockage of the upper respiratory tract in a
sleeping person. With increasing age, about 60% of men and about 40% of women are
affected. In most people, snoring depends on a certain position of the body while
sleeping. Frequently, snoring occurs when sleeping on the back. Snoring can be induced
by different irregularities and causes. There are persons who only snore every now and
then and then there are persons who snore almost every night. If the snoring becomes
too pronounced, even the snoring persons themselves may wake up as a result.
2.2 Information about snoring
The snore stopper is an electric stimulation device that recognises snoring noises from 65
dB (decibel) in its surrounding environment using a microphone sensor if the surrounding
background noises are less than 55 dB (decibel). If the device detects 3 or more
consecutive snoring noises, an electric pulse is activated. When wearing the snore
stopper, your wrist is connected to 2 adhesive electrodes. These adhesive electrodes
transmit the electric pulse from the device through your skin. The intensity of the pulse
may be individually adjusted on the device. With the right settings, you will receive a
weak pulse which will not wake you. However, it is possible that your subconscious will
react to the pulse and lead you to change your sleeping position. Snoring is frequently
caused by a blocked respiratory tract. The snore stopper may contribute to a change of
sleeping position if you snore, making you stop snoring. This means that the snore
stopper can help you to get a good night's sleep.
2.1 How does a snore stopper work?
Warning/Danger: The device must not be used by persons with a
pacemaker!
The instructions must be followed at all times!
Warning/Danger: If not used correctly, serious or fatal injuries and damage
may occur!
Read and follow the instruction manual.
The symbols refer to the following content:
The safety symbols shown in this instruction manual contain information relating to the
correct use of the snore stopper and your safety.
BASIC INFORMATION
1.0 Definition of symbols
2.0 Basic information
GB

5
3.1.1
3.1.2
3.1.3
3.1.4
3.1.5
3.1.6
3.1.7
3.1.8
3.1.9
3.2.0
3.2.1
3.2.3
3.3.1
3.3.2
3.3.3
3.3.4 The sense of intensity may actually depend on the respective daily constitution.
Therefore, the intensity may be adjusted to the personal needs using the intensity
control of the snore stopper.
If not prescribed otherwise by the doctor, we recommend a daily use during the
night while sleeping.
The snore stopper is only intended for external application (skin application) on
humans for electric stimulation.
Only use the snore stopper for the intended use, i.e. for an exterior stimulation-
current and low-frequency therapy on the wrist of the human body.
3.3 Usage/environment for which the snore stopper is suited
Ensure that the snore stopper is not covered by pieces of clothing or other objects
(e.g. pillow) when using the device.
Please retain the instruction manual during the useful life of the product.
Accessories from other devices may not be used.
Misuse and use not in conformity with the application must be avoided.
Please store the instruction manual for later reference and hand over the
instruction manual if you pass on the snore stopper to third parties. Please make
the instruction manual available to third parties. The instruction manual is an
integral part of the snore stopper.
Without having sought prior medical advice, do not use the snore stopper on
areas that hurt inexplicably, swollen muscles or after a serious muscle injury. The
therapy with the snore stopper is not a substitution for a diagnosis and treatment
administered by a medical professional.
Should you have any doubts concerning the therapy with the snore stopper,
please seek medical advice in advance.
Only use the snore stopper while sleeping and do not perform any other activities
while using the device.
Please remove all metallic objects, such as jewellery, belts, watches and other
utensils, before starting the therapy, in order that they do not come into contact
with the device.
If, during the use of the snore stopper, skin alterations, pain, swellings, discomfort
or other anomalies occur, you must stop the therapy immediately and seek
medical advice.
The snore stopper may not be repaired, used or modified (changed) by the users
themselves in case of a defect. If used incorrectly, the stimulation current may
cause pain, injuries and burns.
3.1 General safety instructions
SAFETY INSTRUCTIONS
If, during the usage, anomalies occur, the therapy must be stopped immediately.
3.0 Safety instructions
GB
You must not use the snore stopper in the following circumstances: a. heart
diseases and cardiac arrhythmias (may lead to cardiac arrest),
b. directly on wounds, c. pregnancy, d. in patients with pace-
makers, e. parts of the body with poor circulation, f. in patients with
psychological and/or emotional problems, g. in patients with
diagnosed dementia (deterioration of mental faculties), h. in
patients with a low IQ (intelligent quotient).
6
3.4.1
3.4.2
3.4.3
3.4.4
3.4.5
3.4.6
3.4.7
3.4.8
3.4.9
3.5.1
3.5.2
3.6.1
3.6 Application for which the snore stopper is not suited
The snore stopper is only intended for private use and not for trade or commercial
use.
Only use the snore stopper for the intended use, i.e. for an exterior stimulation-
current and low-frequency therapy on the wrist of the human body. Seek medical
advice concerning therapeutic questions.
3.5 Application for which the snore stopper is suited
Electric medical devices are subject to special precaution measures with regard to
EMC (electromagnetic compatibility). Please observe the applicable EMC
information (page 15-18) pertaining to the installation and use of the device.
Please note that portable and mobile HF (high frequency) communication devices
(e.g. mobile phones) may have an effect on electric medical devices.
The snore stopper is only intended for private use and not for trade or commercial
use.
Maintain a distance of at least 1.5 metres to shortwave or microwave devices
and/or high-frequency surgical devices when using the snore stopper, otherwise
there is a risk of skin irritations or burns caused under the electrodes. Do not use
the snore stopper on mountains when higher than 3,000 metres.
During the use, the snore stopper may interfere with other electric devices or
may be disturbed by other electric devices. Therefore, do not use the snore
stopper near other electric devices.
Do not use the snore stopper near highly flammable substances and gases or
near explosives.
Only use the snore stopper while in bed or sleeping.
Do not use or wear the snore stopper while bathing, showering or in any other
environment with high air humidity. Keep away from any liquids during use.
Injuries may occur or the health may be negatively impacted by an increased
stimulation or a short circuit – mortal danger!
The snore stopper may not be used at the same time with other medical and
electric devices of any kind.
3.4 Usage/environment for which the snore stopper is not
suited
SAFETY INSTRUCTIONS
GB

7
3.6.2
3.6.3
3.7.1
3.7.2
3.7.3
3.8.1
3.8.2
3.8.3
3.8.4
3.8.5
3.8.6
3.8.7
3.8.8
3.8.9 Please remove the protective foil before attaching the adhesive electrodes. The
adhesive strength of the electrodes depends on the condition of the skin, position
and number of applications. If the adhesive electrodes no longer completely stick
on the surface of the skin, they must be replaced with new adhesive electrodes.
The adhesive electrodes must make contact across the entire electrode surface in
order to avoid the formation of isolated current densities, which could lead to
burns to the skin. Replace the protective foil after using of the device.
The snore stopper may not be used on parts of the body where the skin is
inflamed and open and fresh wounds are present.
Every person reacts differently to an electric stimulation. If the therapy is not
successful, please seek medical advice.
The adhesive electrodes are attached to the snore stopper by the adhesive
properties of the electrodes.
Please ensure that the device has been switched off during the application of the
adhesive electrodes and the strapping on and removal of the snore stopper.
Before using the device, the areas of skin intended for the adhesive electrodes
must be thoroughly cleaned and dried. The skin must be greaseless and clean.
Using the snore stopper may result in skin irritations under certain circumstances.
If skin irritations, e.g. redness, blisters or itching, occur, the snore stopper should
no longer be used! Do not use the snore stopper permanently on the wrist, as this
could lead to skin irritations.
If you want to reposition the snore stopper during use, make sure that you switch
off the device first.
The adhesive electrodes may only be attached to the snore stopper. Please ensure
that the device has been switched off during the application or removal of the
adhesive electrodes.
3.8 Using the snore stopper
Keep the snore stopper out of the reach of children. Children could swallow the
small parts and choke. Children could hurt themselves when using the snore
stopper.
The snore stopper must be stored out of the reach of children and adolescents
younger than 18 years of age.
Children may not be treated with this snore stopper.
3.7 Usage by children and adolescents
Do not use the snore stopper if you could hurt yourself as a result of a sudden
fright.
Please consult your doctor before using the snore stopper in the following
circumstances: a. acute diseases, b. sleep disturbances, c. infectious diseases, d.
fever, e. blood pressure problems, f. skin diseases, g. after an accident, h. nausea
or dizziness, i. onset of illnesses, j. if anomalies occur, k. pain of inexplicable
cause, l. diabetes, m. seizures, n. breathing pauses during sleep, o. if pain is not
experienced in some parts of the body, p. during pregnancy, q. persons with
metal and implants in the body.
SAFETY INSTRUCTIONS GB

8
4.0.1
4.0.2
4.0.3
4.1.1
4.2.1
4.2.2
4.2.3
4.2.4
4.3.1
4.3.2
4.3.3
4.3.4
4.3.5 Do not immerse the snore stopper in water or other liquids.
A suitable, commercially available disinfectant may be used for disinfecting the
device. Let the snore stopper dry completely afterwards.
For hygienic reasons, every user should use his/her own adhesive electrodes.
Gently clean the surfaces of the snore stopper with a soft, damp cloth. Use water
to wet the cloth. Use a mild detergent in case of stubborn stains. Ensure that the
snore stopper has been switched off. Therefore, you must always remove the
batteries before cleaning the device. Let the snore stopper dry completely
afterwards. Do not use chemical detergents or abrasive cleaners to clean the
snore stopper or the adhesive electrodes.
The snore stopper must not be exposed to direct sunlight. Do not place the snore
stopper on hot surfaces.
4.3 Cleaning and care of the snore stopper
If the snore stopper ASG 341 is intended for trade or commercial use, a technical
safety inspection is necessary every 2 years in accordance with § 6 MPBetreibV
[German Medical Devices Operator Ordinance]. The technical safety inspection
must be carried out by a company specialised in medical devices. Further
information may be obtained from our service centre (see page 20).
If the device is not used for a prolonged period, remove the batteries from the
device.
Do not dismantle or repair the snore stopper; otherwise, technical or physical
accidents may occur – warning/mortal danger!
The snore stopper is maintenance-free.
4.2 Storage/maintenance of the snore stopper
The adhesive electrodes must not be attached to other parts of the body except
the outside of the respective wrist.
4.1 Where the adhesive electrodes must not be attached
The size of the adhesive electrodes must not be changed, e.g. by cutting off
pieces of the electrodes.
Do not use adhesive electrodes with an electrode size smaller than 20 x 25 mm
(4.8 cm²), as this could lead to the flowing of a too large current density, thereby
possibly causing injuries.
The adhesive electrodes are attached to the inside of the device. Place the snore
stopper against the outside of the wrist. This ensures that the adhesive electrodes
are also pressed against the skin surface on the outside of the wrist. Adjust the
wristband in order that the snore stopper is pressed against the wrist.
4.0 Where the adhesive electrodes must be attached
SAFETY INSTRUCTIONS
3.9.0 Avoid contact between the two electrodes. This will cause a short circuit, during
which an increased current density flows. This increased current density may lead
to burns and injuries.
GB

6.0 Information about noise sources, noise scale up to 70 dB
(decibel)
9
7.1
6.1
If the snore stopper is to be recycled, observe the legal regulations
concerning disposal. Contact your municipality or a waste disposal
company for further information. Dispose of the snore stopper in
accordance with the Waste of Electrical and Electronic Equipment
Directive 2002/96/EC - WEEE.
7.0 Disposal of the snore stopper
Noise sources and possible health effects:
0 dB: Hearing threshold of humans.
10 dB: Rushing of leaves, normal breathing of a human.
20 dB: Quiet garden, whispering, quiet room.
30 dB. Noise from the refrigerator, noise from side streets.
40 dB: Quiet conversation. Sleep disturbances may occur. Learning and
concentration disorders are possible.
50 dB: Normal conversation (approx. 1 m away), ambient noise level.
60 dB: Stress threshold is reached. Noisy conversation.
65 dB: Possible onset of damages of the autonomic nervous system. Increased
risk of cardiovascular disorders.
70 dB: Household noise level, vacuum cleaner.
1 x 1.5 V battery AAA
3 x alcoholic cleaning
wipes
3 x adhesive electrodes
1 instruction manual
1 snore stopper ASG 341
5.0 Scope of delivery/contents of packaging
SCOPE OF DELIVERY/DISPOSAL GB
- Disposal of batteries: Empty batteries must not be disposed of in
the household waste. They must be disposed of through your elec-
tronic dealer or public recycling collection point. You as a consumer
are legally bound to return empty batteries.
- These symbols denote batteries containing hazardous substances:
Pb = contains lead, Hg = contains quicksilver, Cd = contains cadmium.
- If swallowed, batteries can be life-threatening. Therefore, please keep batteries
and products out of reach of small children. If a battery was swallowed, seek
medical help immediately.
- If a battery leaked, avoid contact with skin, eyes and mucous membranes. Rinse
the affected spots extensively with clear water and seek medical help immediately.
- Batteries must not be recharged (except for rechargeable batteries), dismantled,
thrown into fire or short-circuited.
- Protect batteries against excessive heat. Remove the batteries from the product,
if they are empty or the product will not be used for a longer period. This avoids
damage caused by leaking batteries.
- Do not use rechargeable batteries!
10
Pb, Hg, Cd
8.1
8.2
8.3
8.4
8.5
8.6
8.7
8.8
8.9
Image 2
Image 1
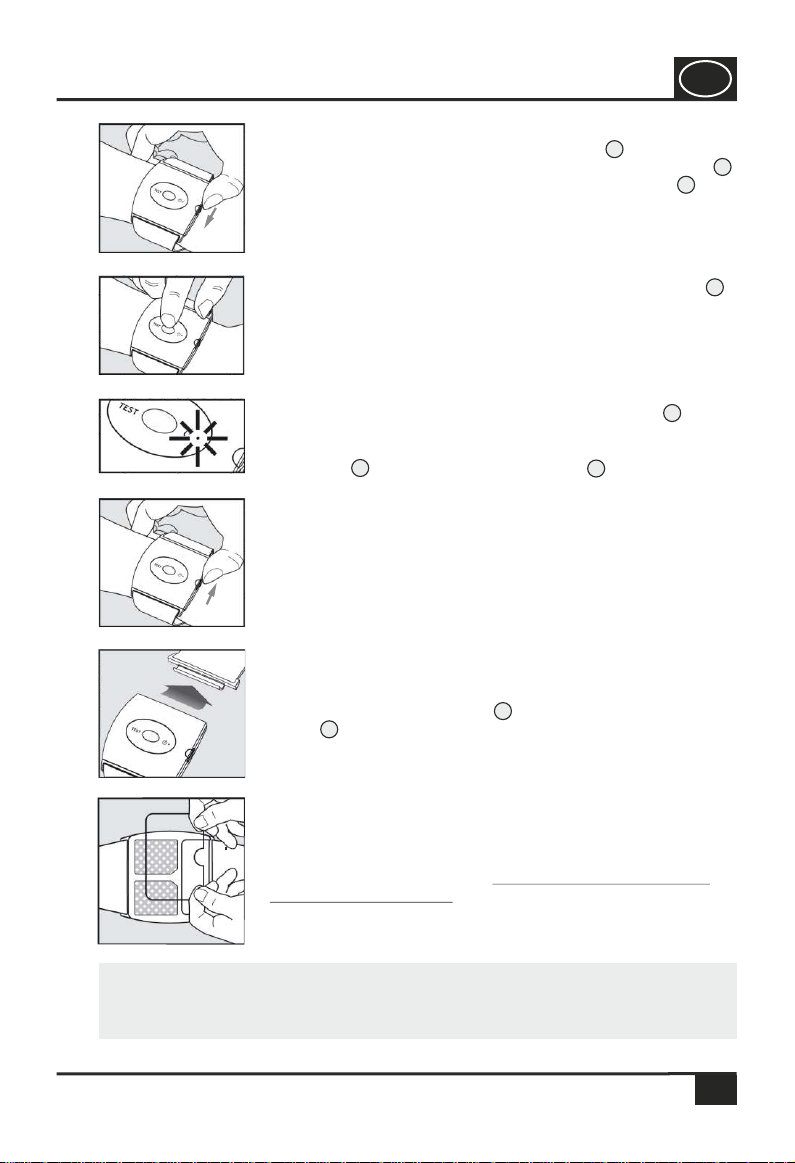
Step 3:
To close the wristband, insert the
wristband holder at the side of the
housing and push it down until it snaps
into place.
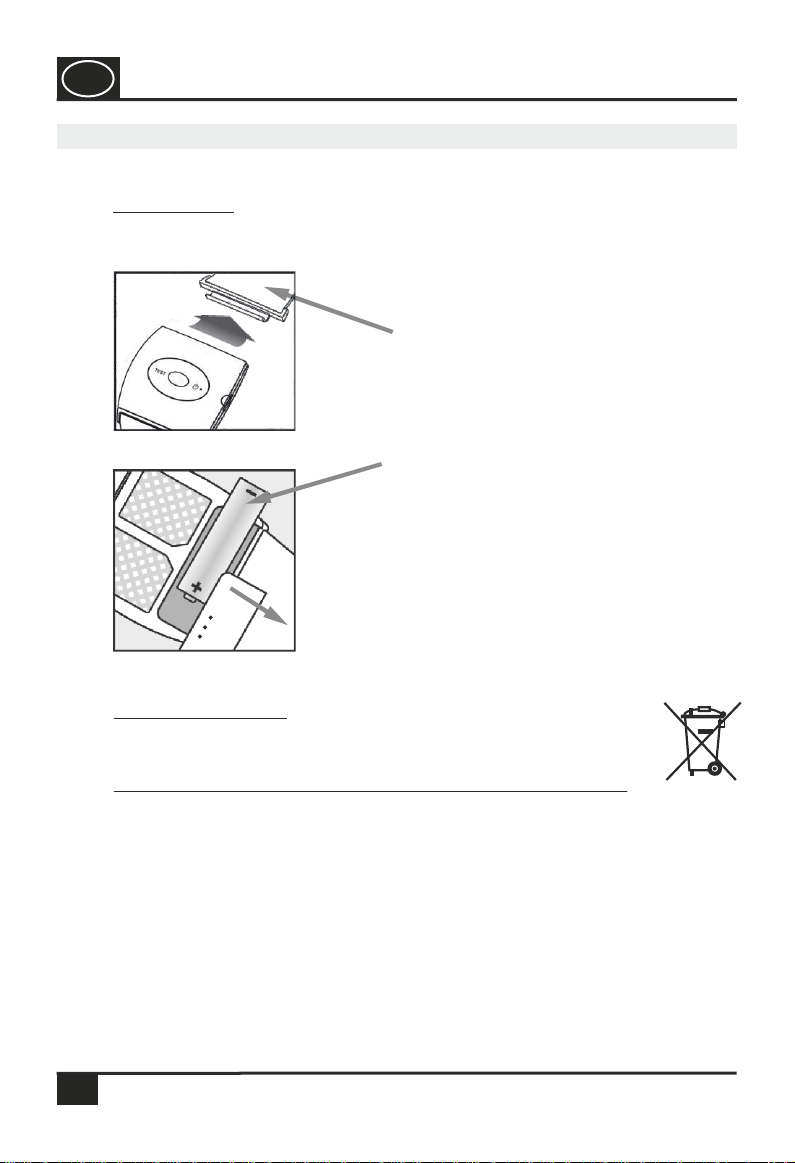
Step 2:
To change the battery, gently push the
battery lid down and slide it away from
the housing. Observe the correct polarity
when inserting the battery. Place the
battery lid on the housing and slide it
towards the device until the battery lid
snaps into place.
Step 1:
Open the wristband by pulling up the
wristband holder on the housing (see
image 1).
Place 1 battery (type AAA) in the device as shown in the figure. Observe the
correct polarity (+ and – pole).
Type of battery: Only use alkaline batteries for the snore stopper. Rechargeable
batteries (batteries: NiMH, NiCd) may not be used.
8.0 Changing the batteries and information about batteries
CHANGING THE BATTERIES
GB

Step 3:
Place the adhesive electrodes with the adhesive outside on
the two black rubber pads on the backside of the device,
so that the surfaces of the black pads and the adhesive
electrodes match (image 7).
Step 1:
Before attaching the adhesive electrodes, clean the two
black electrode pads with the enclosed alcoholic cleaning
wipe (see image 5) or a commercially available alcoholic
cleaning wipe.
12
Step 7:
Place the snore stopper about 3 cm away from the wrist and
press the snore stopper onto the arm (image 11). Insert the
wristband holder again und press down to lock it.
6
Step 6:
Open the two hook and loop fasteners on the outside of the
wristband and unfasten the band until the hook and loop
fastener reaches the wristband holder . Lift off the
wristband holder to unlock it from the housing (image 10).
6
6
Step 8:
Pull at the two ends of the wristband in order to adjust the
device to your lower arm (image 12). If necessary, change
the length of the wristband; if it sits too tightly, this could
impair the blood circulation.
Step 5:
Wait for 15 minutes after the application of the adhesive
electrodes, so that the adhesive layer can achieve optimal
adhesion. After this is done, the protective foil can be
removed (image 9).
Step 4:
Press the adhesive electrodes on the device with both
thumbs using a rotating movement to achieve a better
adhesion of the adhesive electrodes (image 8). Do not
remove the large protective foil!
Step 2:
Remove the blue protective foil from the adhesive electrodes
as shown in image 6. This protective foil will not be needed
for the further application.
APPLICATION OF THE DEVICE
10.0 Application of the snore stopper
Image 5
Image 7
Image 8
Image 9
Image 10
Image 12
Image 11
3 cm
Image 6
Image 5
Image 7
Image 8
Image 9
Image 10
Image 12
Image 11
3 cm
Image 6
Image 5
Image 7
Image 8
Image 9
Image 10
Image 12
Image 11
3 cm
Image 6
5
5
GB
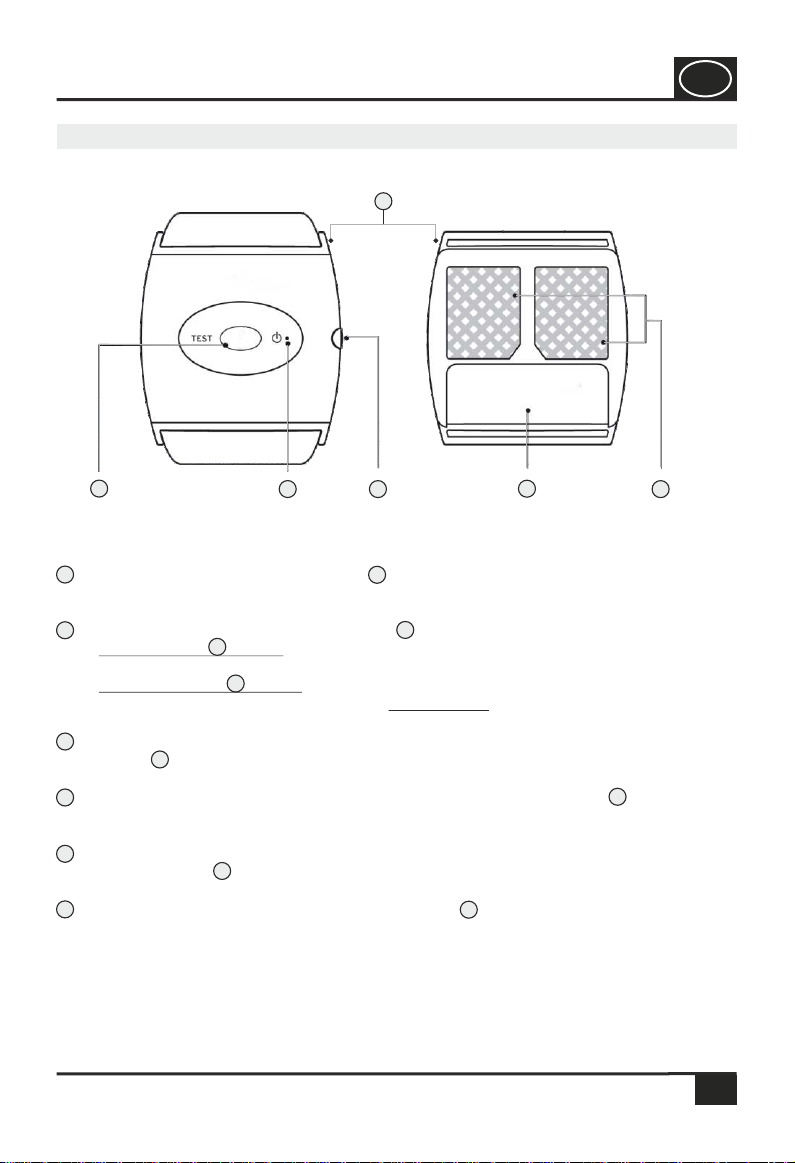
Wristband holder: The two wristband holders can be removed.
Black rubber pads: The adhesive electrodes are attached to the two black
electrode pads .
Battery compartment: By removing the battery compartment lid , the
battery (1 x 1.5 V AAA) can be replaced.
On/off switch: In position “0”, the device is switched off. By turning the on/off
switch , you can adjust the intensity in levels from 1 to 7.
LED lamp: The green or red LED lamp flashes when the device is switched on.
If the red LED flashes, there is no contact between the adhesive electrodes and
the skin surface. The snore stopper is not ready for use.
If the green LED flashes, contact is made between the adhesive electrodes and
the skin surface. The snore stopper is ready for use.
Test button: Press the test button a total of 3 consecutive times to test the
set intensity.
12345
Image 3 Image 4
6
11
1
2
4
5
3
6
1
2
2
2
3
4
5
6
9.0 Designation and function of the snore stopper
DESIGNATION/FUNCTION GB
Note: The sense of intensity may actually depend on the respective daily constitu-
tion. Therefore, the intensity may be adjusted to the personal needs by the user.
The snore stopper also reacts to snoring noises above 65 dB (decibel) emitted from
third parties if the background noise in the room is below 55 dB (decibel).
Step 13:
Open the hook and loop fastener on the outside of the
wristband, loosen the band until the hook and loop fastener
reaches the wristband holder . Lift off the wristband
holder to unlock it from the housing (image 17).
Step 11:
If the device sends an electric pulse, the green LED
flashes during the duration of the pulse (image 15). If the
snore stopper detects a noise above 65 dB (decibel), the
green LED flashes once. If the red LED flashes, there
is no skin contact with the device. Please repeat step 9.
Step 10:
To test the strength of intensity, press the test button
three times in a row. The device activates an electric
pulse for about 5 seconds. If you feel that the intensity is
too low, increase and test the intensity gradually to
adjust it to your needs.
Step 9:
Switch on the device. Turn the on/off switch to intensity
level 2 (image 13). If skin contact is good, the green LED
flashes once. If skin contact is not good, the red LED
flashes once (image 15). In this case, tighten the band a
little and repeat step 9 until the green LED lights up.
13
Image 13
Image 14
Image 15
1
2
2
2
3
2
2
Image 16
Image 18
Image 17
6
6
Step 14:
Gently remove the snore stopper from the wrist. Now place
theprotective foil on the adhesive electrodes (image 18). The
adhesive electrodes will not get dirty and the useful life is
prolonged as a result of this. After 8 hours, the device will
switch off automatically. If the snore stopper will not be used
for a prolonged period, please remove the battery.
Step 12:
Please note that the device is set to the required intensity
level during the sleep phase. To end the therapy you must
first set the intensity button to “0”. Switch off the device
after waking up and remove the snore stopper from the
wrist (see step 13).
APPLICATION OF THE DEVICE GB
14
Fault
Cause
Solution
The batteries are inserted,
but there is no signal from
the LED lamp.
There could be foreign
objects in the battery
compartment. Ensure that
the batteries are full and are
inserted with the correct
polarity. Check if the battery
contacts make contact.
If foreign objects are
present, they must be
removed.
Replace the battery with a
full one and observe the
correct polarity.
There is a defect in the
electronic components.
Remove the battery and
replace it again after approx.
3 seconds.
The green LED flashes, but
the adhesive electrodes do
not transmit any current
pulses.
The adhesive electrodes are
not attached correctly or no
longer make contact with
the skin surface.
Check the adhesive
electrodes. If necessary,
replace with new adhesive
electrodes.
An intensity level is set on
the device, but there is only
a low stimulation felt
through the adhesive
electrodes.
The battery is not strong
enough.
Replace the battery with a
full one and observe the
correct polarity.
The skin surface is not clean.
Clean the skin surface.
The entire adhesive face of
the electrodes does not stick
anymore and is exhausted.
The adhesive electrodes
must be replaced with new
ones.
The stimulation current
intensity increases, even
though a low intensity was
selected.
The adhesive electrodes are
not fully attached to the skin
surface.
Adjust the wristband so that
the adhesive electrodes are
pressed onto the skin
surface.
The adhesive electrodes only
partially stick to the skin
surface.
The adhesive electrodes are
used up and must be
replaced with new ones.
The device stops while in
use.
The battery no longer has
enough power.
Replace the battery with a
full one and observe the
correct polarity.
There is a defect in the
electronic components.
Remove the battery and
replace it after approx. 3
seconds.
The skin surface shows
alterations or is red.
It is possible that the skin
alterations are caused by the
adhesive electrodes.
Stop the therapy
immediately and seek
medical advice.
11.0 Technical problems, troubleshooting
TECHNICAL PROBLEMS
GB

15
Guidelines and manufacturer's declaration – electromagnetic emissions
The model ASG 341 is intended for use in an environment as specified below. The customer
or the user of the model ASG 341 should ensure that it is used in such an environment.
Electromagnetic
interference
measurements
Compliance
Electromagnetic environment – guideline
HF emissions
according to CISPR 11
Group 2
The model ASG 341 only uses HF energy for
its internal operation. Therefore, its HF
emissions are very low, which most probably
do not cause any malfunctions in nearby
electronic installations.
HF emissions
according to CISPR 11
Class B
The model ASG 341 is intended for use in all
facilities, including residential environments
and such environments that are directly
connected to the public power supply, which
also supplies buildings that are used for
residential purposes.
Harmonic current
emissions according
to IEC 61000-3-2
Not applicable
Emission of voltage
fluctuations/flicker
according to IEC
61000-3-3
Not applicable
Table 1 – Instruction and manufacturer's specifications – electromagnetic emissions – for all
INSTALLATIONS and SYSTEMS (see 6.8.3.201 a) 3).
Instruction and manufacturer's specifications – electromagnetic emissions
The (INSTALLATION or the SYSTEM) is designed for the use in the electromagnetic
environment described below. The customer or the user of the (INSTALLATION or the
SYSTEM) should ensure that it is used in such an environment.
Emission test
Compliance
Electromagnetic environment –
instruction
HF emissions
CISPR 11
Group 2
The (INSTALLATION or the SYSTEM) only uses
HF energy for its internal operation.
Therefore, only very low HF emissions occur,
which most probably do not cause any
malfunctions in nearby electronic installations.
12.0 Information regarding electromagnetic immunity
ELECTRIC IMMUNITY GB
16
Guidelines and manufacturer's declaration – electromagnetic immunity
The model ASG 341 is intended for operation in an electromagnetic environment as specified
below. The customer or the user of the model ASG 341 should ensure that it is used in such
an environment.
Immunity tests
IEC 60601 –
test level
Conformity
level
Electromagnetic environment –
guidelines
Electrostatic
discharge immunity
test according to
IEC 61000-4-2
± 6 kV contact
discharge
± 8 kV air
discharge
Not applicable
± 8 kV air
discharge
Floors should be made of wood or
concrete or furnished with ceramic
tiles. If the floor is furnished with
synthetic material, the relative air
humidity must be at least 30%.
Electrical fast
transient/burst
immunity according
to IEC 61000-4-4
± 2 kV for power
cables
± 1 kV for input
and output cables
Not applicable
The quality of the supply voltage
should correspond to the voltage
of a typical commercial or hospital
environment.
Surges according to
IEC 61000-4-5
± 1 kV differen-
tial mode voltage
± 2 kV common
mode voltage
Not applicable
The quality of the supply voltage
should correspond to the voltage
of a typical commercial or hospital
environment.
Voltage dips, short
interruptions and
voltage variations
according to IEC
61000-4-11
< 5% UT (>95 %
dip of UT) during
½ period 40% U
T
(60% dip of UT)
during 5 periods
70% UT (30% dip
of UT) during 25
periods < 5% UT
(> 95% dip of UT)
during 5 s
Not applicable
The quality of the supply voltage
should correspond to the voltage
of a typical commercial or hospital
environment. If the user of the
model ASG 341 requires a
continuous function also when
interruptions in the energy supply
occur, it is recommended to supply
the model ASG 341 from an
uninterruptible power source or a
battery.
Magnetic fields at
the power frequency
(50/60 Hz)
according to IEC
61000-4-8
3 A/m
3 A/m
Magnetic fields at the power
frequency should correspond to
typical values that can be found in
a commercial or hospital
environment.
NOTE U is the alternating mains voltage prior to application of test levels.
T
ELECTRIC IMMUNITY
12.0 Information regarding electromagnetic immunity
GB
17
Guidelines and manufacturer's declaration – electromagnetic immunity
The model ASG 341 is intended for operation in an electromagnetic environment as specified
below. The customer or the user of the model should ensure that it is used in such an
environment.
Immunity
tests
IEC 60601 –
test level
Conformity
level
Electromagnetic environment –
guidelines
Portable and mobile radio devices should
not be used in closer proximity to the
[device or system] including the cables as
the recommended protective distance
calculated in accordance with the formula
for the respective transmission frequency.
Recommended protective distance:
Conducted HF
disturbances
according to
IEC 61000-4-6
3 Vrms
150 kHz to 80
Mhz
3 Vrms
d = 1.2
Radiated HF
disturbances
according to
IEC 61000-4-3
3 V/m
80 MHz to 2.5
Ghz
3 V/m
d = 1.2 80 MHz to 800 MHz
d = 2.3 800 MHz to 2.5 GHz
Where P is the rated power of the
transmitter in watt (W) according to the
specification of the transmitter
manufacturer and d is the recommended
protective distance in metres (m).
According to an on-siteainvestigation, the
field strength of stationary radio
transmitters is in all frequencies lower than
the conformity level.b
Disturbances are possible in the vicinity of
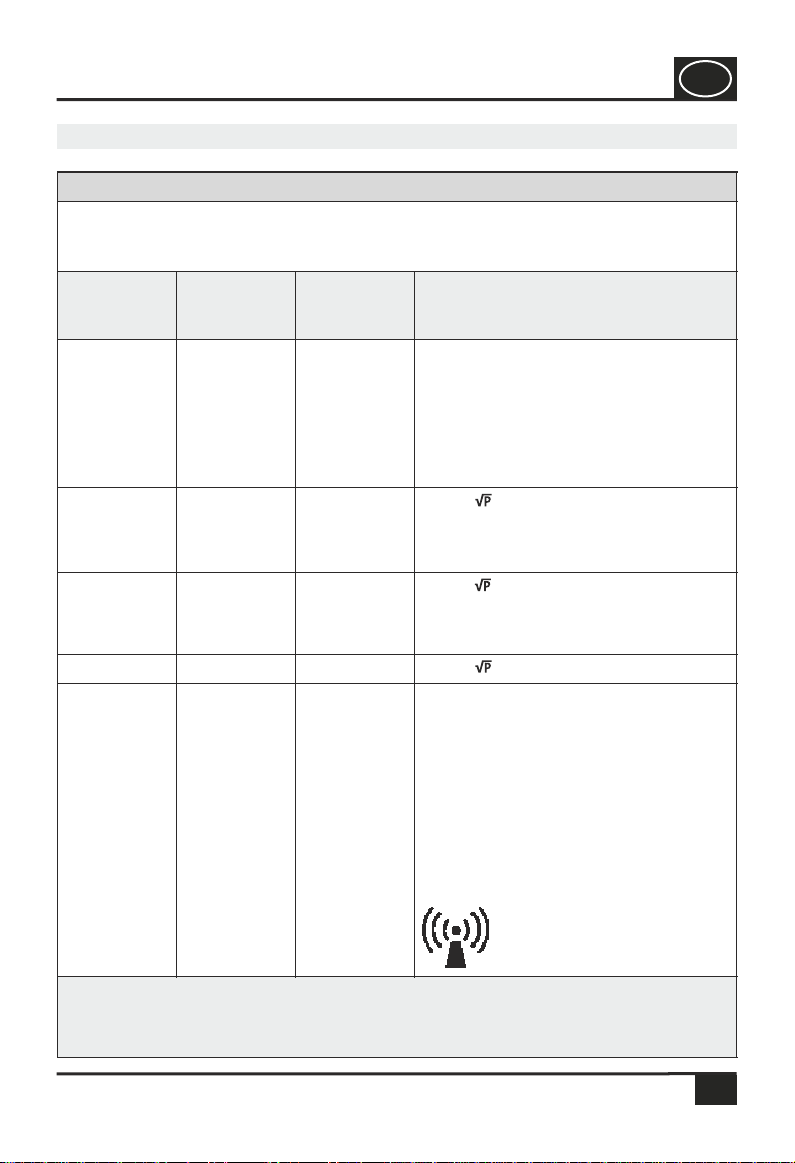
devices carrying the following symbol.
NOTE 1 For 80 MHz and 800 MHz, the higher value is applicable.
NOTE 2 These guidelines might not apply to all situations. The spreading of electromagnetic
waves is influenced by absorptions and reflections of buildings, objects and people.
ELECTRIC IMMUNITY
12.0 Information regarding electromagnetic immunity
GB

18
a. Theoretically, the field strength of stationary transmitters, such as base stations of wireless
telephones and land mobile services, amateur radio stations, AM and FM radio and television
stations, cannot be predicted precisely. An investigation of the site is recommended to
determine the electromagnetic environment due to stationary HF transmitters. If the
determined on-site field strength of the model ASG 341 exceeds the conformity level specified
above, the normal operation of the model ASG 341 must be observed at every application
site. If unusual performance characteristics are observed, additional measures might have to
be taken, such as reorientation or relocation of the model ASG 341. b. Not applicable above
the frequency range from 150 kHz to 80 Mhz.
Recommended protective distances between portable and mobile HF telecommunication
devices and the [DEVICE or the SYSTEM].
The model ASG 341 is intended for operation in an electromagnetic environment in which the
HF disturbances are controlled. The customer or user of the model ASG 341 may contribute to
the avoidance of electromagnetic disturbances by observing the minimum distance between
portable and mobile HF telecommunication devices (transmitters) and the model ASG 341,
depending on the output rating of the communication device as specified below.
Rated power of the
transmitter W
Protective distance depends on the transmission frequency m
150 kHz to 80 Mhz
d=1.2 P
80 Mhz to 800 Mhz
d=1.2
800 Mhz to 2.5 Ghz
d=2.3
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1
1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
For transmitters whose rated power is not specified in the table above, the distance can be
caculated with the help of the formula of the respective column, with P being the rated power of
the transmitter in watt (W) in accordance with the specification of the transmitter manufacturer.
NOTE 1 For the calculation of the recommended protective distance of transmitters in the
frequency range from 80 MHz to 2.5 GHz, an additional factor of 10/3 was used to minimise
the possibility that a mobile/portable communication device unintentionally brought into the
patient area could lead to disturbances.
NOTE 2 These guidelines might not apply to all situations. The spreading of electromagnetic
waves is influenced by absorptions and reflections of buildings, objects and people.
ELECTRIC IMMUNITY
12.0 Information regarding electromagnetic immunity
GB

Model type:
Dimensions (LxWxH):
Weight:
Adhesive electrode surface:
Material:
Device rating plate:
Electric specifications:
Power supply:
Pulse voltage (V):
Frequency (HZ):
Pulse width (duration):
Electric tolerances:
LED facilities:
Output channel:
Automatic shut-off:
Application
specifications:
Ambient temperature:
Max. air humidity at
normal operation:
Atmospheric pressure:
Storage/transport
specifications:
Atmospheric pressure:
Snore stopper ASG 341
Housing approx. 65 x 60 x 15 mm
Approx. 45 g incl. weight of battery
2 pieces with 20 x 24 mm (4.8 cm²)
Synthetic materials, metal
Lot designation,
Serial number, 00001 (consecutive number)
Date of manufacture,
2013-04 (year, month)
The snore stopper ASG 341 is certified in accordance with
the EU directive 93/42 EEC concerning medical devices.
Manufacturer: Handelshaus Dittmann GmbH,
Kissinger Straße 68, D-97727 Fuchsstadt/Germany
Protection against electric shock according to type BF
(body float). An application device of type BF with a higher
protection against electric shock on the body, however, not
directly on the heart.
1.5 V DC, 1 x AAA battery (V= volt, DC= direct current)
5.0 to 33 volt at a load of 1000 ohm
50 Hz (vibrations per second)
800 µs (microseconds)
+/- 20% at a load of 1000 ohm
LED lamps comply with class I
1 channel with adjustable intensity
After about 8 hours
19
V2913ASG341
2013-04
0123
-10°C
50°C
10%
85%
Snore stopper ASG 341
Battery: 1.5V DC, 1 x AAAbattery
Handelshaus Dittmann
GmbH, D-97727
Fuchsstadt/Germany
V2913ASG341
0123
2013-04
TECHNICAL SPECIFICATION/SYMBOLS
Storage/transport temperature:
-10°C - 50°C (degree Celsius)
Max. air humidity during storage and transport:
10% - 85% (percent)
700 hPa – 1060 hPa (hectopascal)
10°C - 40°C (degree Celsius)
30% - 85% (percent)
700 hPa – 1060 hPa (hectopascal)
13.0 Technical specification, symbols, pictograms
GB

Copyright © Handelshaus Dittmann GmbH, 2013
Handelshaus Dittmann GmbH
Kissinger Straße 68
D-97727 Fuchsstadt/Germany
Manufacturer:
Yours sincerely
Handelshaus Dittmann GmbH
Abteilung Service-Center
Kissinger Straße 68
D-97727 Fuchsstadt/Germany
E-mail: hotline@servicecenter.tv
Telephone hotline: +49 (0) 180-6000228 (€ 0.20 per call from a German landline
number, max. € 0.60 per call from a German mobile phone networks)
www.dittmann-gmbh.com
Please contact the service centre in the event of a complaint concerning the
snore stopper ASG 341!
If necessary, the service centre will arrange for the collection of the device.
Only PREPAID parcels are accepted by the service centre!
NOTE:
Do not hesitate to contact us if technical problems, questions and warranty claims
concerning this snore stopper ASG 341 arise:
After the expiration of the warranty period you may send the defective snore stopper
ASG 341 to the address specified below for repair. Repairs after the expiration of the
warranty period are subject to charge.
The warranty does not include:
- damage due to incorrect usage
- defects that were already known to the customer when purchasing the product
- wear and tear parts
- damage due to unauthorised interventions and personal negligence on the customer's
part
The statutory warranty period is 24 months as of the purchase date for material and
manufacturing defects of the product. Please keep the receipt as proof of the purchase of
the snore stopper ASG 341 in order to be able to assert a possible warranty claim.
The has been developed and manufactured with great care.snore stopper ASG 341
20
TERMS OF WARRANTY
14.0 Terms of warranty
GB
Table of contents
Other Dittmann Medical Equipment manuals