Dittmann IHG 375 User manual

IHG 375
GB
1
0123
•
•
•
•
•
•
•
•
•
•
•
Version 1, 2013-04
Ultrasound Inhaler IHG 375
Micro-fine atomisation of the inhalation solution by a
special misting technology
Application possible in various positions
Portable device, in a handy size for inhaling medication
during the day
Noiseless operation
Easily handled with a one-button function
Device is easily cleaned
Battery-operated with 2 x AA batteries
Mains-operated, possible with a 230 V / 3 V DC adapter
(not included)
24-month warranty
Contents: 1 Inhaler IHG 375, 1 flexible mask for adults,
1 flexible mask for children, 1 mouthpiece, 1 connecting
piece, 1 storage bag, 1 Instructions for Use, 2 x 1.5 V AA-
batteries
Instructions for Use
Inhaler IHG 375

1.0
2.0
2.1
2.2
3.0
3.1
3.3
3.4
3.5
3.6
3.7
3.8
4.0
4.1
4.2
4.3
4.4
5.0
6.0
7.0
8.0
9.0
10.0
11.0
12.0
13.0
4
4
4
4
5
5
6
6
7
7
7
8
8
9
9
9
10
11
12
12
13
14
15
15
16
17
2
GB
Measures to be taken after having used the Inhaler
Advice on how to use the Inhaler
Using the Mask
Using the Mouthpiece
Starting to use the Inhaler IHG 375
Designations / Functions of the Inhaler IHG 375
Changing Batteries and Advice on Batteries
Disposing of the Inhaler IHG 375
Delivery Extent / Contents of Box
Operation with a Power Supply Unit (optional)3 V / DC
Hygiene measures
Storage / Maintenance of the Inhaler IHG 375
Which medicines are not suitable for inhalation?
Which medicines are suitable for inhalation?
Advice on the of the Inhaler IHG 375application
Usage by children and youths
For which application area is the Inhaler IHG 375 not suitable?
For which application area is the Inhaler IHG 375 suitable?
For which is the Inhaler IHG 375 not suitable?usage / environment
For which usage / environment is the Inhaler IHG 375 suitable?
General Safety Instructions
Safety Instructions
Information about the application of the Inhaler IHG 375
What are the properties of the Inhaler IHG 375?
Basic Information
What do the symbols mean?
No. Subject Page
CONTENTS

14.0
14.1
14.2
15.0
16.0
17.0
18.0
18
18
18
19
20
24
25
3
Dear Customer,
may we congratulate you for having purchased the Inhaler IHG
375 and express our thanks for your trust in our product. In order
to guarantee your inhaler to function optimally and maintain its
performance, we kindly ask you to read the instructions for use
before using the inhaler for the first time. Thus it is ascertained
that you will enjoy this product for a long time.
Warranty
Technical Data, Symbols, Pictograms
Information regarding electromagnetic immunity
Technical Malfunctions, Rectifying Problems
Disinfecting the Inhaler and its Accessories
Cleaning the Inhaler and its Accessories
Cleaning and Disinfecting the Inhaler IHG 375 and the Accessories
No. Subject Page
CONTENTS GB

4
The Inhaler IHG 375 is suited for the treatment of
the respiratory tract illnesses, such as e.g. asthma
or allergies by using liquid medication and other
medical remedies. In the appliance's atomiser the
liquid is transformed into an aerosol by a special
ultrasound technology that - due to its properties -
can easily be inhaled, distributed and absorbed into
the upper and lower respiratory tract. In case there
is not enough liquid available, the device switches off
automatically. It can be operated either with two AA-batteries
or with a 3 V power supply unit (optional). The device is suitable
to be used at home. Inhaling liquid medicines and other medical
remedies should only take place under medical instructions and
in a quiet and relaxed situation. When using the device, breathe in slowly and deeply
so that the aerosol can be distributed into the finest bronchial tubes, and subsequently
breathe out normally.
2.2 Information about the application of the Inhaler IHG 375
The Inhaler IHG 375 is an inhaling device for atomising liquid medicines and other
medical remedies. By being atomised, medication and medical remedies approved for the
inhalation therapy can be absorbed by the respiratory tract in form of aerosols.
2.1 What are the properties of the Inhaler IHG 375?
2.0 Basic Information
Warning about the danger of infection: The infection can be caused by
viruses, bacteria, fungi or the like!
This advice should be followed implicitly!
Warning/Danger: Improper use may cause serious injuries, damage and be
life threatening!
Read and follow the instructions for use!
The symbols represent the following:
The safety symbols shown in these instructions for use give advice on the intended use
of the inhaler and also to ensure your safety.
1.0 What do the symbols mean?
BASIC INFORMATION
GB

3.1.1
3.1.2
3.1.3
3.1.4
3.1.5
3.1.6
3.1.7
3.1.8
3.1.9
3.2.0
3.2.1
3.2.2
3.2.3
3.2.4
3.2.5
3.2.6 Be careful that the aerosol from the atomiser does not get into contact with your
eyes. Depending on the medicine, it is possible that the aerosol may have a
damaging effect on your eyes.
Always observe the generally required hygiene measures when using the inhaler.
Take care that no liquid medication or other medical remedies run or drip onto or
into the inhaler or the power unit.
Never clean the basic casing and the power supply unit under running water or
other liquids and never immerse them into any liquids!
Never leave the inhaler and power supply unit in reach of children or persons who
need to be looked after!
Clean and disinfect the inhaler after every application. Instructions for cleaning
and disinfection can be found on Page 18.
Take care not to subject the inhaler to hard impacts or even drop it.
Always check the inhaler for malfunctions prior to using it. Should you find a fault
resp. a defect, do not use the device.
Do not deposit heavy or sharp-edged objects on the inhaler or its accessories.
Only use the device together with the attached accessories or original spare
parts. Never use accessories from different appliances.
In order to avoid damage and dangers, improper use and not using the device in
accordance with the instructions fur use must be avoided!
Keep these instructions for use in a safe place for the period of the product's life
span and for future reference. Also pass these instructions on with the device,
should you give it to a third person. Make the instructions for use available to third
persons. The instructions for use are part of the inhaler.
It is imperative that you should ask your doctor about the type, dosing and
application of liquid medicines and other medical remedies that are best suited for
you to inhale with this device.
Should you have any questions or doubts about using the inhaler, ask your doctor
prior to using the device. Using the inhaler does not replace a medical diagnosis
and treatment!
Should irregularities become apparent at the inhaler, stop using the device at
once and consult your doctor.
In case of a malfunction the inhaler must not be used, dismantled, repaired by
yourself or modified (changed). Damage can be caused by using the inhaler
incorrectly.
3.1 General Safety Instructions
3.0 Safety Instructions
SAFETY INSTRUCTIONS GB
5

3.4.1
3.4.2
3.4.3
3.4.4
3.4.5
3.4.6
3.4.7
3.4.8
3.3.1
3.3.2
3.3.3
3.3.4
6
Electric medical devices are subject to special safety measures with regard the
electromagnetic compatibility (EMC). Therefore, please observe the EMC-advice
( ) when installing and using the device.Pages 20 - 23
Remember that portable and mobile high-frequency communication devices (e.g.
mobile phones) may influence electric medical appliances.
The inhaler is designed for personal use, not, however, for being used
commercially.
Do not use the inhaler at a distance of less than 1.5 m to a short-wave- or
microwave appliance resp. a high frequency-HF-surgical appliance. Do not use the
inhaler in the mountains above 2000 m.
The inhaler can influence other electric appliances negatively or be influenced by
other electric appliances. Therefore do not use the inhaler in the vicinity of other
electric appliances.
Do not use the inhaler in the vicinity of highly inflammable substances and gases
or explosives.
Do not use the inhaler while having a shower, swimming, in the sauna or in other
surroundings with a high air humidity. Keep away any kind of liquid during the
application.
The inhaler must not be used at the same time as other medical and electrical
appliances of any kind.
3.4 For which is the Inhaler
IHG 375 not suitable?
usage / environment
If not otherwise prescribed by the doctor, we recommend an average
period of treatment of approximately 15 minutes up to three times daily.
This inhaler is a medical appliance. Only use liquid medicines and other medical
remedies that are suitable for this device and only according to the instructions
and prescriptions issued by your doctor. Always follow the application- and safety
advice of the medicines or other remedies!
A treatment with the inhaler does not replace medical advice or treatment!
Always ask your doctor first in case you are in pain or have an illness.
Use the inhaler exclusively for the intended purpose: for inhaling suitable liquid
medicines and other medical remedies via the respiratory tract of a person. Any
other use is considered to be improper and may lead to damages or even
compromise your health. The manufacturer is not liable for damages that have
been caused by improper or incorrect use.
3.3 For which usage / environment is the Inhaler IHG 375
suitable?
SAFETY INSTRUCTIONS
GB

7
3.6.1
3.6.2
3.7.1
3.7.2
3.7.3
3.5.1
3.5.2
3.5.3
Do not let children handle the inhaler. The small parts could be swallowed by
children and lead to suffocation. Children may also injure themselves while
handling the device.
Keep the inhaler out of reach of children and youths below 18.
Children and youths under the age of 18 should always be supervised when using
the inhaler.
3.7 Usage by children and youths
In case of a hyper-sensitivity of the respiratory tract, liquid medicines and
medicinal remedies containing essential oils may cause a spasmodic constriction
of the bronchi with breathing problems (bronchospasm). Ask your doctor!
Essential medicinal plant oils, gargle solutions, liquids and solutions for rubbing in,
or for steam baths must not be used in connection with the Inhaler IHG 375;
these are too viscous and cannot be atomised. They may also damage the device.
3.6 For which area is the Inhaler IHG 375
not suitable?
application
As the device can be tilted up to an angle of approximately 45°, it can also be
used in a lying down position, e.g. or for a child being held in one's arm.
The portable, easily handled device operates noiselessly, and with its simple
inhalation application is particularly suitable to be used at home.
The device can be used for an inhaling therapy in case of an acute and chronic
disease of the respiratory tract. Due to the finely sprayed aerosol, the active
ingredients of the atomised liquid medicines or medical remedies are distributed
very well in the respiratory tract and thus prevent illnesses to manifest
themselves in the respiratory tract or to soothe or heal already existing
complaints.
3.5 For which application area is the Inhaler
suitable? IHG 375
SAFETY INSTRUCTIONS GB

8
3.8.1
3.8.2
3.8.3
3.8.4
3.8.5
3.8.6
3.8.7
3.8.8
3.8.9
3.9.0
3.9.1
3.9.2
3.9.3
4.0.1
4.0.2 This inhaler is a medical appliance. Only use liquid medicines and other medical
remedies that are suitable for this device and only according to the instructions
and prescriptions issued by your doctor and always follow the application- and
safety advice of the medicines or other remedies!
The inhaler is suitable for liquid medicines or medicinal remedies that are used for
preventing and treating acute and chronic illnesses of the upper and lower
respiratory tract. The medicine is atomised in the device. The thus produced fine
aerosol, the active ingredients of the liquid medicines or medicinal remedies are
easily distributed in the respiratory tract and in that way act preventative or, in
case of already existing illnesses will soothe or support the healing process of the
disease.
4.0 Which medicines are suitable for inhalation?
Do not carry out any other activity while using the inhaler. Make sure that you use
the inhaler in a calm and relaxed atmosphere.
Do not use the inhaler in an ambient temperature of above 40 °Celsius! There
are further details about the permissible ambient conditions in Chapter 17.0 on
Page 24.
Take care that the device is disconnected from the power supply before being
cleaned.
When using the device with the power supply unit, always disconnect the power
supply unit from the power supply and the inhaler upon finishing the application!
Before connecting the power supply unit, ascertain that the mains voltage V (Volt)
and the frequency in Hz (Hertz) of the adapter complies with that of the power
supply.
Do not connect the power supply unit (optional) of the inhaler to the 230 V power
supply with wet or damp hands!
Do not cover the air inlets of the device as this may prevent the air from
circulating.
Only use the inhaler with liquid medication and medicinal remedies! Never use
other liquids of any kind for the device as this could damage your health and / or
also damage the device!
Due to hygiene reasons, each user should only use his own accessories (e.g.
mouthpiece and inhaling mask).
In case the application with the inhaler is not successful, consult your doctor in
any case.
Always switch the inhaler off when installing and de-installing accessories, or
filling the medication container.
Avoid shaking the inhaler while in use. This could lead to a malfunction and the
liquid could leak out.
Before using the device take care that the components and accessories (e.g.
mouthpiece and mask) have been assembled in accordance with these instructions
for use (see Page 14 -15).
3.8 Advice on the application of the Inhaler IHG 375
SAFETY INSTRUCTIONS
GB

9
4.1.1
4.1.2
4.1.3
4.1.4
4.2.1
4.2.2
4.2.3
4.2.4
4.2.5
4.2.6
4.2.7
4.2.8
4.3.1
4.3.2 Also follow the advice of Chapter 14 on regarding cleaning and
disinfecting the device and its accessories.
Page 18
The device and also the accessories must be thoroughly cleaned and disinfected
before their initial use, after a longer period of storage as well as after every
application!
WARNING: Danger of infection through viruses, bacteria and germs!
Infections can transmit life-threatening diseases! The device must be
cleaned and disinfected after every application.
By adhering to these measures the risk of infection can be minimised.
4.3 Hygiene Measures
Always use and store the device in relevant ambient conditions (temperature and
air humidity) in accordance with our instructions on Page 24.
Never try to dry the device or its components in a microwave oven!
Protect the device from direct sunlight, impacts, heat, open fire as well as any
contact with corrosive liquids! Never place the device on a hot surface!
Store the device as well as its accessories in a dry, clean and safe place.
In case the inhaler IHG 375 is used commercially a safety-technical inspection
becomes necessary in compliance with § 6 MPBetreibV (Medical Devices Operator
Ordinance) every 24 months. The safety-technical inspections must be carried out
by a specialist company for medical products. Further information can be obtained
from our Service Center (see .Page 25)
Remove the batteries from the device, if it is not used for a longer period of time.
Do not dismantle or repair the device as this could cause technical accidents or it
may also cause injuries. Warning! Danger of injuries!
The Inhaler IHG 375 is maintenance-free.
4.2 Storage / Maintenance of the Inhaler IHG 375
Never inhale aerosols consisting of a mixture of different medicines – unless this
has explicitly been discussed with your doctor and he has prescribed this.
Never use liquid medicines and medicinal remedies in your inhaler without
consulting your doctor beforehand and getting advice from him!
In case of a hyper-sensitivity of the respiratory tract, liquid medicines and
medicinal remedies containing essential oils may cause a spasmodic constriction
of the bronchi with breathing problems (bronchospasm). Ask your doctor!
Essential medicinal plant oils, gargle solutions, liquids and solutions for rubbing
in, or for steam baths must not be used in connection with the Inhaler IHG 37;
these are too viscous and cannot be atomised. They may also damage the device.
4.1 Which medicines are not suitable for inhalation?
SAFETY INSTRUCTIONS GB

4.3.3
4.3.4
4.3.5
4.3.6
4.3.7
10
Remove the batteries first, before connecting the
device to the power supply unit.
Remove the power supply unit from the device
and the 230 V mains after every application.
The Inhaler IHG 375 can also be operated with a
suitable AC/DC power supply unit with a voltage
of 3 Volt and 1.0 A (Ampere). Before connecting
the power unit to the 230 V mains, make sure
that the voltage V (Volt) and the frequency Hz
(Hertz) match.
Attention!
While being connected to the power supply unit,
there must not be any batteries in the device!
Also, rechargeable batteries must not be charged
in the device while the inhaler is connected to the
power supply unit! There is an immediate danger
of being injured through an explosion and also
damage caused to objects!
4.4 Operation with a Power Supply Unit 3 V / DC (optional)
Empty the medication container after every application, and make sure that there
is no residual liquid left in the device!
Never immerse the inhaler into water or other liquids.
Mouthpiece, connecting piece and atomiser cover can be disinfected by being
boiled. Place the parts in a steriliser or in a saucepan with boiling water, immerse
the parts completely into water and boil them for about 20 minutes. Disinfecting
by boiling must not be used for the atomiser unit, atomiser head with metal
mesh, basic unit and masks. A usual suitable disinfectant can be used for
disinfecting these parts, if necessary. Subsequently, leave the parts and the
device to dry out thoroughly.
Due to hygiene reasons and in order to avoid passing on any infection, each user
should use his own accessories. This applies to the atomising unit, atomiser cover,
connecting piece, mouthpieces and above all the masks, which cannot be
disinfected by being boiled!
Clean the surfaces of the inhaler gently with a soft, slightly damp cloth; take care
that no water can penetrate the device. In case of a heavier soiling you can use a
mild detergent. The inhaler must, however, not be switched on during the
cleaning process. To this end remove the batteries from the device prior to each
cleaning process. Subsequently leave the inhaler to dry out thoroughly. Do not
use any chemical cleaning agents or abrasive products to clean the inhaler.
The inhaler should not be switched on during cleaning and servicing.
SAFETY INSTRUCTIONS
GB

11
1 x Storage Bag
2 x 1,5 V AA Batteries
1 x Mouthpiece
1 x Connecting Piece
1 x Mask for Adults
1 x Mask for Children
1 x Instructions for Use
1 x Inhaler IHG 375
Please check that all parts have been delivered upon unpacking the parts and also check
for damages. Do not use the device in case of any apparent damage, or if the
instructions for use are missing!
5.0 Delivery Extent / Contents of Box
DELIVERY EXTENT GB

6.1
7.1
7.2
7.3
7.4
7.5
7.6
7.7
7.8
Pb, Hg, Cd
12
Fig. 1 Fig. 2
Disposing of batteries: Empty batteries do not belong into the house-
hold waste. Dispose of them through your electric appliances trader
or take them to the public recycling collection point. You, as a con-
sumer, are legally obliged to return empty batteries.
These signs indicate hazardous batteries:
Pb = contain lead, Hg = contain mercury, Cd = contain cadmium.
If swallowed, batteries may be life threatening. Therefore, keep batteries
and products out of reach of children. In case a battery was swallowed,
it is imperative that a doctor is consulted at once.
In case a battery has leaked, avoid contact with skin, eyes and mucous
membranes. Rinse the affected areas with lots of clear water and consult a doctor
immediately or seek medical help.
Batteries must not be recharged (apart from re-chargeable batteries), be
dismantled, put into an open fire or short-circuited.
Protect the batteries from excessive heat. Remove the batteries from the product,
once they are empty or you do not use the product for a longer period of time.
Thus you avoid damage that may occur by leaking.
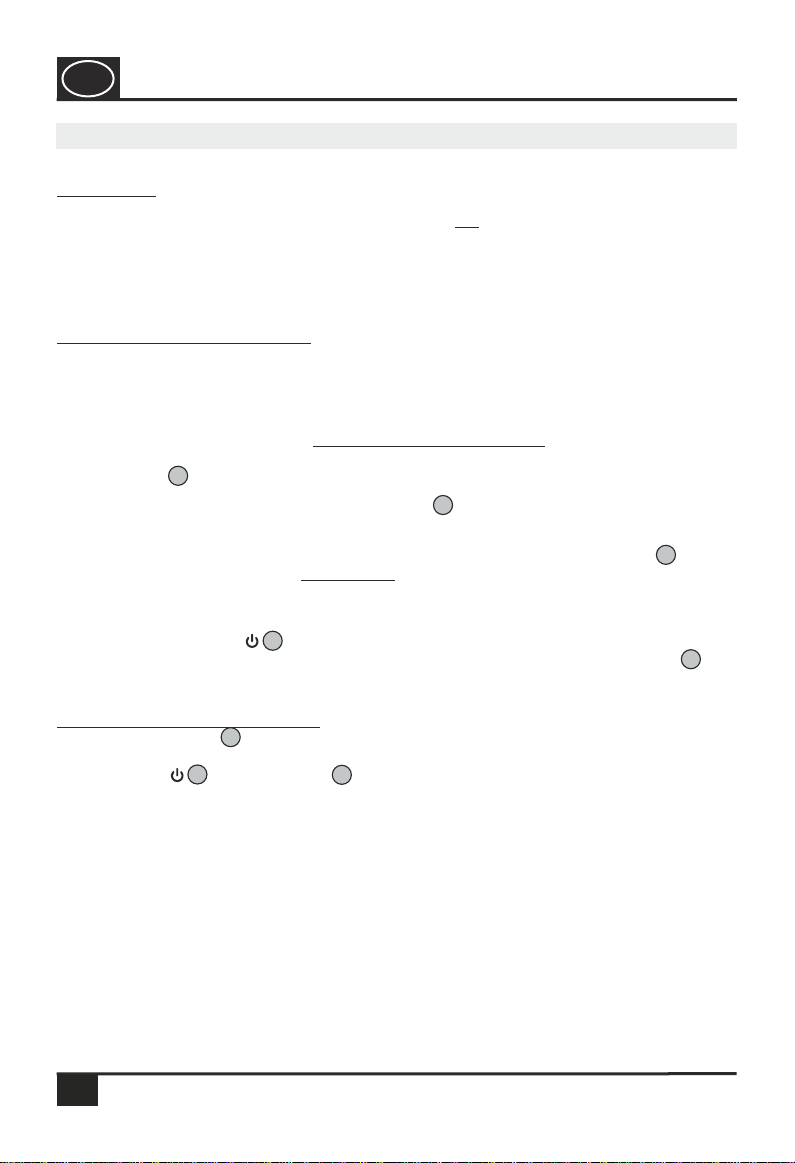
For opening the cover press the locking clip towards the batteries and open the
battery recess according to Fig. 1. Remove the empty batteries and replace them
with two new 1.5 V AA batteries (Fig. 2). Observe the correct polarity when
inserting the batteries (see marking / indentation +/-on the interior surface of
the battery recess cover). Close the cover and press it down, until it engages
firmly.
Battery recess cover
Insert the 2 x 1.5 V AA batteries into the device by observing the correct
polarities (+and -Pole).
Types of batteries: For the Inhaler IHG 375 you require 2 AA batteries. Do not use
rechargeable batteries!
7.0 Changing Batteries and Advice on Batteries
In case the Inhaler IHG 375 is to be recycled, the disposal must follow
the legal regulations. To this end ask your municipal offices or a waste
disposal specialist company. Dispose of the inhaler in compliance with
the EU-Guideline 2002/96/EG-WEEE on old electric- and electronic
appliances.
6.0 Disposing of the Inhaler IHG 375
DISPOSAL/CHANGING BATTERIES
GB

13
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15.
16.
12
13
14
15
11
1
2
3
4
16
5
7
8
6
10
9
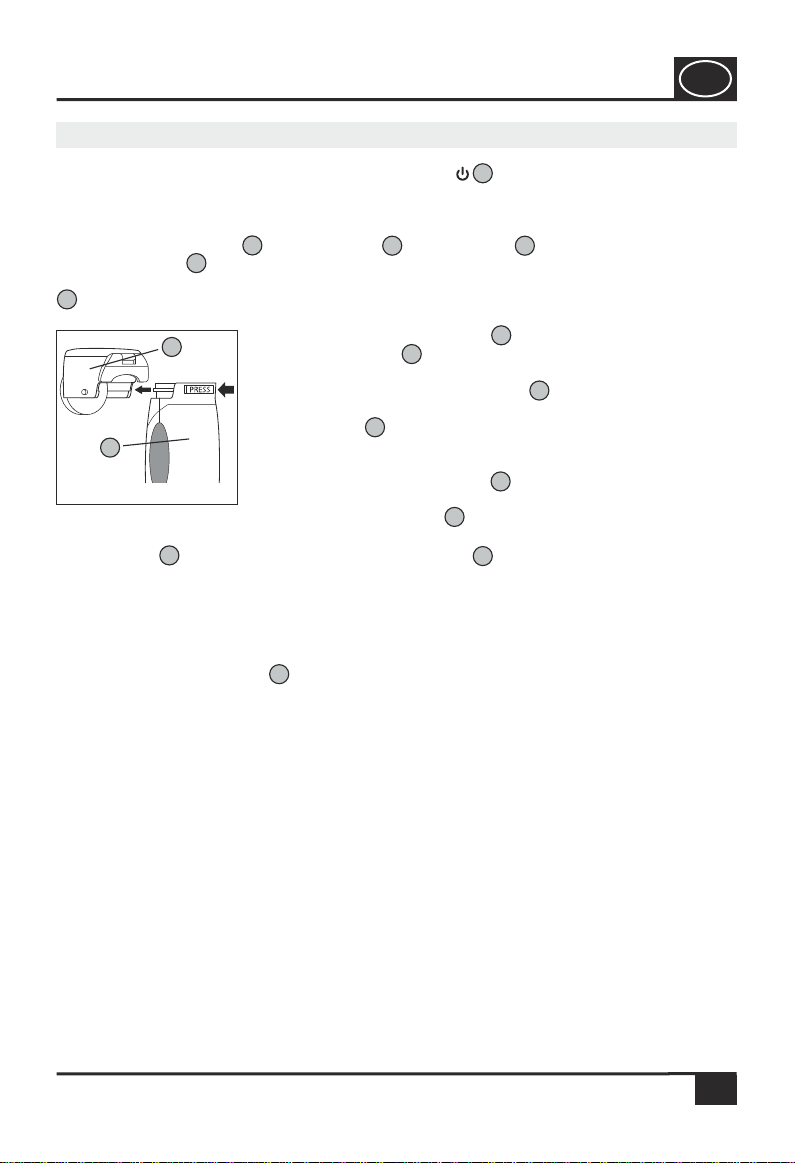
Atomiser Unit
Medication Container
Container Cover with Locking
Atomiser Head with Metal Mesh
Contacts/Electrodes for the Atomiser Unit
Basic Unit/Basic Casing of the Inhaler
LED (green) indicating the operational readiness
LED (yellow) indicating weak batteries
ON/OFF Switch
Battery Recess Cover
Connection bush for Power Supply Unit 3 V DC with Cover (on the back of
the device)
Atomiser Cover
Mask for Adults
Connecting Piece
Mask for Children
Mouthpiece
8.0 Designations / Functions of the Inhaler IHG 375
FUNCTIONS OF THE DEVICE GB

Step 4
Now hold the basic unit in the left hand and open the
atomiser unit by loosening the locking at the container
cover of the atomiser with the thumb and index finger of
your right hand and by lifting the cover of the medication
container towards the top (see Fig. 6).
Step 5
Pour the liquid medication into the container for liquids next to
the atomiser head (see Fig. 7); a minimum of 0.5 ml and a
maximum of 8 ml can be added. In order to increase or reduce
the temperature of the added liquid, hot or cold water can be
filled into the chamber next to the container for the liquid
medicines.
Step 3
Remove the atomiser cover according to Fig. 5 in reverse
order to Step 2.
Step 2
Take the basic unit into your left hand and slide the bottom
part of the atomiser cover onto the basic unit in the
direction of the arrow, according to Fig. 4.
A sound signal (Ka) will indicate that the atomiser cover is
properly installed on the atomiser unit .
Attention! The inhaler must not be switched on! Store the
thus assembled device in the dry and clean storage bag. For
the start-up: Continue with Step 3!
14
Fig. 3
Fig. 4
Fig. 5
Fig. 6
Fig. 7
Fig. 8
6
6
12
12
1
12
1
2
12
3
3
1
3
9.0 Starting to use the Inhaler IHG 375
Step 1
Take the basic unit in one hand and the atomiser unit in
the other hand and slide the atomiser unit onto the basic
unit in the direction of the arrow, according to Fig. 3.
A sound signal (Ka) will indicate that the atomiser unit is
properly installed on the basic unit.
Attention! The inhaler must not be switched on!
6
611
4
Step 6
Close the cover of the medication container according to
Fig. 8 and press the locking at the container cover of the
atomiser unit towards to bottom with the thumb of your
right hand, until a sound signal (Ka) indicates that the cover of
the medication container is properly locked.
Step 7
Replace the atomiser cover on the device (see Step 2). The
device is now ready for operation.
START-UP
GB

Step 2
Now adjust the mask such that it rests lightly over the
patient's nose and mouth. Pull the elastic band of the mask
over the head so that the mask is positioned safely (see
Fig. 12). Press the ON/OFF switch . The green LED
lights up and the device starts operating. Inhale the atomised
liquid medicine by slowly and deeply breathing in through the
nose, enabling the aerosol to be distributed in the nose and
pharynx and the respiratory tract. Breath out normally through
the mouth. Press the ON/OFF switch again to switch off
the device. The green LED will go out.
Step 1
Install one of the masks (for adults) or (for children), by
placing the connecting piece in the direction of the arrow
onto the connection piece of the atomiser cover as shown in
Fig. 11, and subsequently push the connection piece of the
mask into the connecting piece in the direction of the arrow.
The mask for adults and the mask for the children are
used for inhaling atomised liquid medication (aerosols)
through the nose resp. the nose and pharynx.
Step 2
Now insert the mouthpiece into your mouth and hold it with
your lips. Press the ON/OFF switch .
The green LED lights up and the device starts operating.
Inhale the atomised liquid medication (see Fig. 10) by slowly
breathing in deeply through the mouth so that the aerosol can
be distributed in the mouth and pharynx right down to the
finest bronchial tubes. Breath out normally through your nose.
Press the ON/OFF switch again to switch off the device.
The green LED goes out.
The mouthpiece included in the delivery extent is used for
inhaling atomised liquid medicines (aerosols) through the
mouth resp. the mouth and pharynx.
16
12
13
14
15 13 15
13 15
14
14
12
16
12
15
7
9
9
7
9
9
7
7
or
Fig. 9
Fig. 10
Fig. 11
Fig. 12
ADVICE: If required, it is possible to switch from the mouthpiece to the mask during the
application.
11.0 Using the Mask
10.0 Using the Mouthpiece
APPLICATION
Step 1
Install the mouthpiece by placing it in the direction of the
arrow on the connecting piece of the atomiser cover , as
shown in Fig. 9. 12
16
GB
WARNING OF LOW BATTERIES: As soon as the batteries of the device are becoming
weak, the yellow LED lights up as a warning. Keep fresh batteries in stock to be able
to exchange them in case they are required. When you switch on the device with the
switch , the green LED , however, does not light up, it is possible that the
batteries are completely flat. Exchange them for new batteries in this case (see Chapter
7.0 on ).
ON/OFF
Page 12
Press the ON/OFF switch , should you wish to stop or interrupt the treatment. As
soon as the liquid medication or the medicinal remedy in the medication container is
used up, the device switches off automatically.
If there is only little liquid medication or medicinal remedy left in the container , you
can tilt the device towards you (by max. 45°) to make use of the remaining liquid. Do
not shake the device during application as this may lead to a malfunction resp. an
automatic switching off or even to the liquid leaking out.
Take care that the device is tilted at the most by an angle of 45° during application. If
the device is titled further it is possible that the liquid medication leaks out of the
atomiser head .
16
4
4
2
2
9
8
97
If the device is tilted such that the atomiser head has no contact to the liquid
medication any more, the device switches off automatically after approx. 10 seconds.
In order to guarantee the best possible effect, it is recommended to carry out an
inhalation application in a quiet and relaxed atmosphere and with comfortable clothes,
by breathing in slowly and deeply, if possible, so that the aerosol can reach the remote
parts of the respiratory tract. Should you wish to use the inhaler in bed, you should place
a pillow under your back to support the spine and keep it straight. An uncomfortable
body posture and irregular breathing may make inhaling more difficult and block the
respiratory tract.
Do not swallow the inhaled aerosol and take care that the aerosol reaches the places in
the respiratory tract that are the cause of the complaints.
Use the mouthpiece such that the inhaled aerosol is not sprayed onto the tongue.
Exchange defective or worn parts (e.g. masks, mouthpieces, connecting pieces) only for
original parts.
12.0 Advice on how to use the Inhaler
APPLICATION
GB
under clean water for about 1 - 2 minutes after each application. Take care that the
atomiser unit and the contacts for the atomiser unit are always kept clean!
Now empty the residual medication or medicinal remedy liquid
from the medication container and subsequently clean the
device according to the instructions on Page 18. In order to
clean the atomiser head it is recommended to hold the part
Now remove the atomiser unit from the device. To this end
hold the basic unit in your left hand and press the button
PRESS with your left thumb towards the arrow (see Fig. 13).
Then take hold of the atomiser unit with your right hand at
both sides of the lower end and carefully pull this away from
the basic unit .
Remove the mouthpiece resp. the mask (for adults) or (for children) and the
connecting piece from the device in reverse order as described on Chapter
10.0, Step 2 and 11.0, Step 2) after each application. Then remove the
Page 15 (
atomiser cover
from the device.
Always switch the device off with the ON/OFF switch after an application. When
using the device with the power supply unit (optional), always remove this from the
device and from the 230 V power supply after application.
1
6
17
1
6
1
6
4
1
15
9
16 13 15
14
12
2
Fig. 13
ADVICE: The atomiser unit has a life span of approx. 6 months (in case of 3
applications per day). You can purchase spare parts from your specialist trader.
ATTENTION: Never touch the metal mesh of the atomiser head with your hands, or
pointed or hard objects! this may damage the metal mesh!
13.0 Measures to be taken after having used the inhaler
APPLICATION GB

Rinse the atomiser cover , mouthpiece , mask / , and connecting piece
approx. 10 minutes in a 0.1% ammonium chloride solution. Subsequently rinse the parts
under clean water and dry them with medicinal gauze. Clean the metal mesh of the
atomiser head carefully with a medicinal swab soaked in medical alcohol. Then rinse
the atomiser head with clear water and dry the surface carefully with medicinal swabs
(just dab!).
The mouthpiece , connecting piece , and the atomiser cover can be disinfected by
boiling. Place these parts in a steriliser or a saucepan with boiling water such that all
parts are completely submerged and boil for approx. 20 minutes. Dry the accessories with
medicinal gauze. Disinfection by boiling must not be used for the atomiser unit ,
atomiser head with metal mesh , basic unit , masks / , adapter, and storage
bag.
Always remove all accessories after each application (including atomiser unit ,
atomiser cover , mouthpiece and mask / with connecting piece ) from the
device.
Open the container cover of the atomiser unit and drain the residual liquid medication
or medicinal remedies from the container . In order to clean the atomiser head , it
is recommended to rinse the device about 1 - 2 minutes under clear water after each
application.
Subsequently carefully clean the accessories (atomiser unit , atomiser cover ,
mouthpiece , mask / and connecting piece ). The basic unit should not be
rinsed but only wiped with a damp and then with a dry gauze. Dry the accessories
immediately after cleaning with medicinal gauze. It is important that the metal mesh in
the atomiser head is only touched very carefully and not with your bare hands, sharp
or hard implements. Dry the water inlet and water outlet of the atomiser head
carefully with cotton swabs. Store the device and its accessories in a clean bag after
cleaning.
18
1
1
12
12
16
16
13 15 14
14
4
3
44
12
12
16
16 13 15
13 15
14
14
4
44
61
13 15
2
6
14.2 Disinfecting the Inhaler and its Accessories
14.1 Cleaning the Inhaler and its Accessories
WARNING: Danger of infection through viruses, bacteria and germs!
Infections can transmit life-threatening diseases! The device must be
cleaned and disinfected after every application. By adhering to these
measures the risk of infection can be minimised.
14.0 Cleaning and Disinfecting the Inhaler
IHG 375 and the Accessories
CLEANING AND DISINFECTION
GB

Malfunction
Cause
Solution
The device only generates
very little atomised spray.
The yellow LED display for
low batteries lights up.
Replace the batteries with
new ones. Observe the
correct polarity!
The atomiser unit is
contaminated.
Clean the atomiser unit
according to the instructions
of Page 18.
The green LED does not
light up. The device does not
function.
The batteries have been
inserted with the wrong
polarities.
Check, whether the batteries
have been inserted with the
correct polarities into the
battery recess.
The battery voltage is too
low.
Replace the batteries with
new ones. Observe the
correct polarity!
The power unit (optional)
has not been connected
properly to the bush (3 V)
of the inhaler and / or the
power plug (230 V) has not
been connected properly.
Connect the power unit
correctly to the 3 V bush at
the device and / or to the
230 V power supply.
The atomiser unit is not
installed properly.
Install the atomiser unit
properly in accordance with
the instructions of Page 14.
The green LED lights up
but the device does not
work.
The atomiser unit is not
installed properly.
Install the atomiser unit
properly in accordance with
the instructions of Page 14.
The atomiser is heavily
contaminated.
Clean the atomiser unit
according to the instructions
of Page 18.
The liquid medication
does not come into contact
with the atomiser head .
Tilt the device slightly
towards you, enabling the
liquid to collect at the
atomiser head .
Bubbles are forming
between the liquid
medication and the
atomiser head.
Gently shake the liquid
medication in the container
of the inhaler.
The contact poles at the
casing are soiled.
Clean the contacts
with a dry cloth.
11
55
44
19
8
7
7
15.0 Technical Malfunctions, Rectifying Problems
TECHNICAL MALFUNCTIONS GB
20
ELECTRIC INTERFERENCE IMMUNITY
Guidelines and manufacturer's declaration – electromagnetic emissions
The model IHG 375 is intended for use in an environment as specified below. The customer or
the user of the model IHG 375 should ensure that it is used in such an environment.
Electromagnetic
interference
measurements
Compliance
Electromagnetic environment – guideline
HF emissions
according to CISPR 11
Group 2
The model only uses HF energy for
its internal operation. Therefore, its HF
emissions are very low, which most probably
do not cause any malfunctions in nearby
electronic installations.
IHG 375
HF emissions
according to CISPR 11
Class B
The model is intended for use in all
facilities, including residential environments
and such environments that are directly
connected to the public power supply, which
also supplies buildings that are used for
residential purposes.
IHG 375
Harmonic current
emissions according
to IEC 61000-3-2
Not applicable
Emission of voltage
fluctuations/flicker
according to IEC
61000-3-3
Not applicable
Table 1 – Instruction and manufacturer's specifications – electromagnetic emissions – for all
INSTALLATIONS and SYSTEMS (see 6.8.3.201 a) 3).
Instruction and manufacturer's specifications – electromagnetic emissions
The (INSTALLATION or the SYSTEM) is designed for the use in the electromagnetic
environment described below. The customer or the user of the (INSTALLATION or the
SYSTEM) should ensure that it is used in such an environment.
Emission test
Compliance
Electromagnetic environment –
instruction
HF emissions
CISPR 11
Group 2
The (INSTALLATION or the SYSTEM) only uses
HF energy for its internal operation.
Therefore, only very low HF emissions occur,
which most probably do not cause any
malfunctions in nearby electronic installations.
16.0 Information regarding electromagnetic immunity
GB
Table of contents
Other Dittmann Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual
















