Page 3of 35
Table of Contents
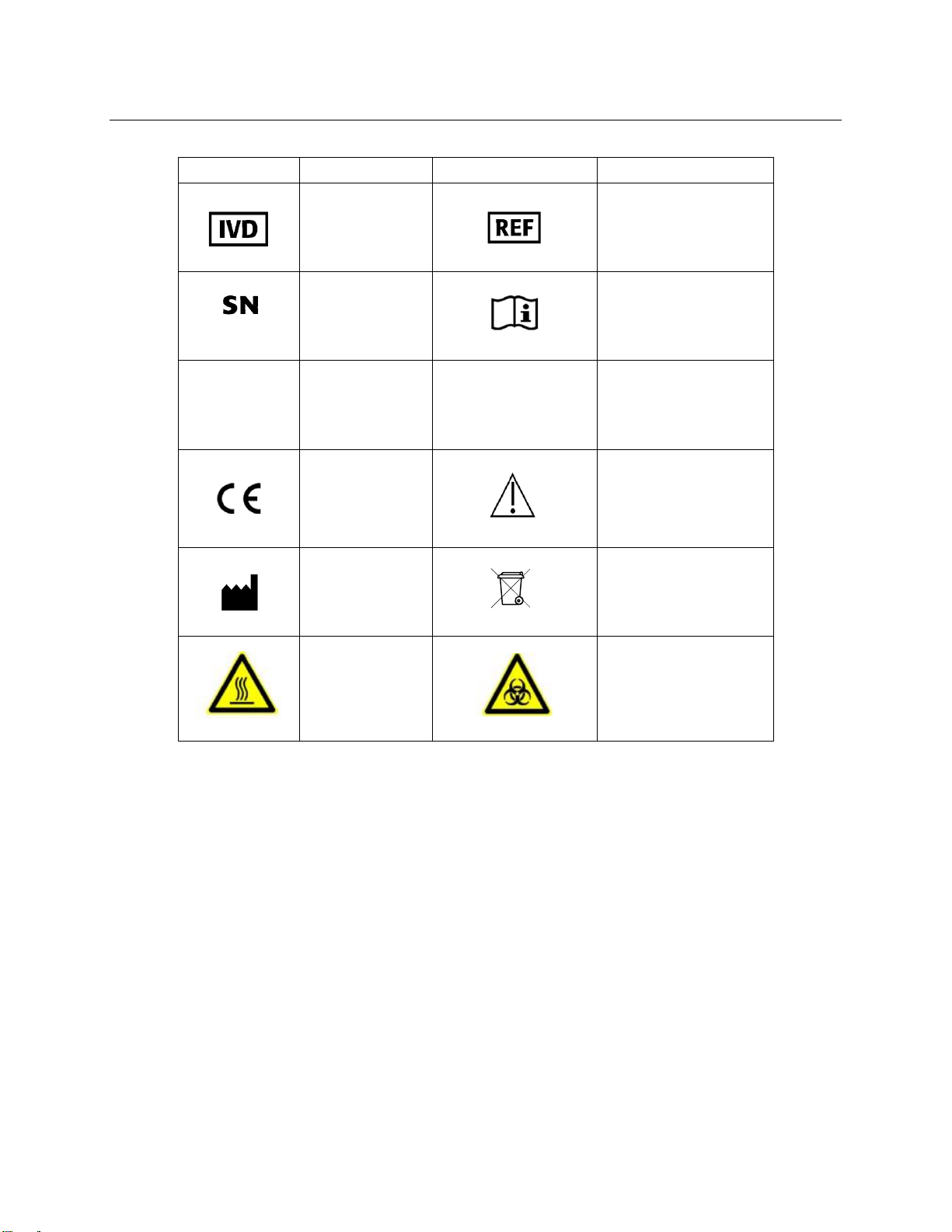
Symbols List ........................................................................................... 4
1. About this manual................................................................................. 5
2. About DxDATATM ................................................................................... 5
2.1 Intended Use................................................................................... 5
2.2 Specifications and performance ............................................................ 6
3. Details of DxDATATM............................................................................... 7
3.1 Principle of detection ........................................................................ 8
3.2 DxDATA TM components....................................................................... 7
4. Storage and Operation of the Equipment.....................................................11
4.1 Environmental conditions and placement instructions .................................11
5. Software Description ............................................................................12
5.1 Software Overview...........................................................................12
5.2 System startup .............................................................................12
5.3 Log in........................................................................................13
5.4 Initialization check ........................................................................14
5.5 Main window ...............................................................................15
5.6 Run window ................................................................................15
5.7 Sample window ............................................................................17
5.8 Results window ............................................................................21
5.9 Settings window ...........................................................................24
5.10 Start initialization.......................................................................30
5.11 Shut down ................................................................................31
6. Instrument warnings and precautions .......................................................32
6.1 Precautions.................................................................................32
7. Troubleshooting ................................................................................33
8. Instrument maintenance ......................................................................35
8.1 Weekly maintenance......................................................................35
8.2 Monthly maintenance .....................................................................35
8.3 Recommended regular maintenance ...................................................35
8.4 Replacing the internal printer paper ...................................................35