32
Chapter 1 : INTRODUCTION
EXPLANATION OF EMS
Electrical Muscle Stimulation is an internationally accepted and
proven way of treating muscular injuries. It works by sending
electronic pulses to the muscle needing treatment; this causes the
muscle to exercise passively.
It is a product derived from the square waveform, originally invented
by John Faraday in 1831. Through the square wave pattern it is
able to work directly on muscle motor neurons. The AGF-6X2 Digital
EMS has low frequency and this in conjunction with the square
wave pattern allows direct work on muscle groupings. This is
being widely used in hospitals and sports clinics for the treatment
of muscular injuries and for the re-education of paralyzed muscles,
to prevent atrophy in affected muscles and improving muscle tone
and blood circulation.
HOW EMS WORKS
1. Relaxation of muscle spasms
2. Prevention or retardation of disuse atrophy
3. Increasing local blood circulation
4. Muscle re-education
5. Immediate post-surgical stimulation of calf muscles to prevent
venous thrombosis
6. Maintaining or increasing range of motion
The EMS units send comfortable impulses through the skin that
stimulate the nerves in the treatment area. W hen the muscle
receives this signal it contracts as if the brain has sent the signal
itself. As the signal strength increases, the muscle f lexes as in
physical exercise. Then when the pulse ceases, the muscle relaxes
and the cycle starts over again, (Stimulation, Contraction and
Relaxation.) Powered muscle stimulators should only be used under
medical supervision for adjunctive therapy f or the treatment of
medical diseases and conditions.
IMPORTANT SAFETY INFORMATION!
Read instruction manual before operation. Be sure to comply with
all “CAUTIONS” and “W ARNINGS” in the manual. Failure to follow
instructions can cause harm to user or device.
Chapter 2: CAUTIONS
1. Federal law (USA) restricts this device to sale by or on the
order of a physician
2. Safety of powered muscle stimulators for use during pregnancy
has not been established.
3. Caution should be used for patients with suspected or diagnosed
heart problems.
4. Caution should be used for patients with suspected or diagnosed
epilepsy.
5. Caution should be used in the presence of the following:
a. When there is a tendency to hemorrhage following acute
trauma or fracture;
b. Following recent surgical procedures when muscle
contraction may disrupt the healing process;
c. Over the menstruating or pregnant uterus; and
d. Over areas of the skin which lack normal sensation.
6. Some patients may experience skin irritation or hypersensitivity
due to the electrical stimulation or electrical conductive medium.
The irritation can usually be reduced by using an alternate
conductive medium, or alternate electrode placement.
7. Electrode placement and stimulation settings should be based
on the guidance of the prescribing practitioner.
8. Powered muscle stimulators should be kept out of the reach of
children.
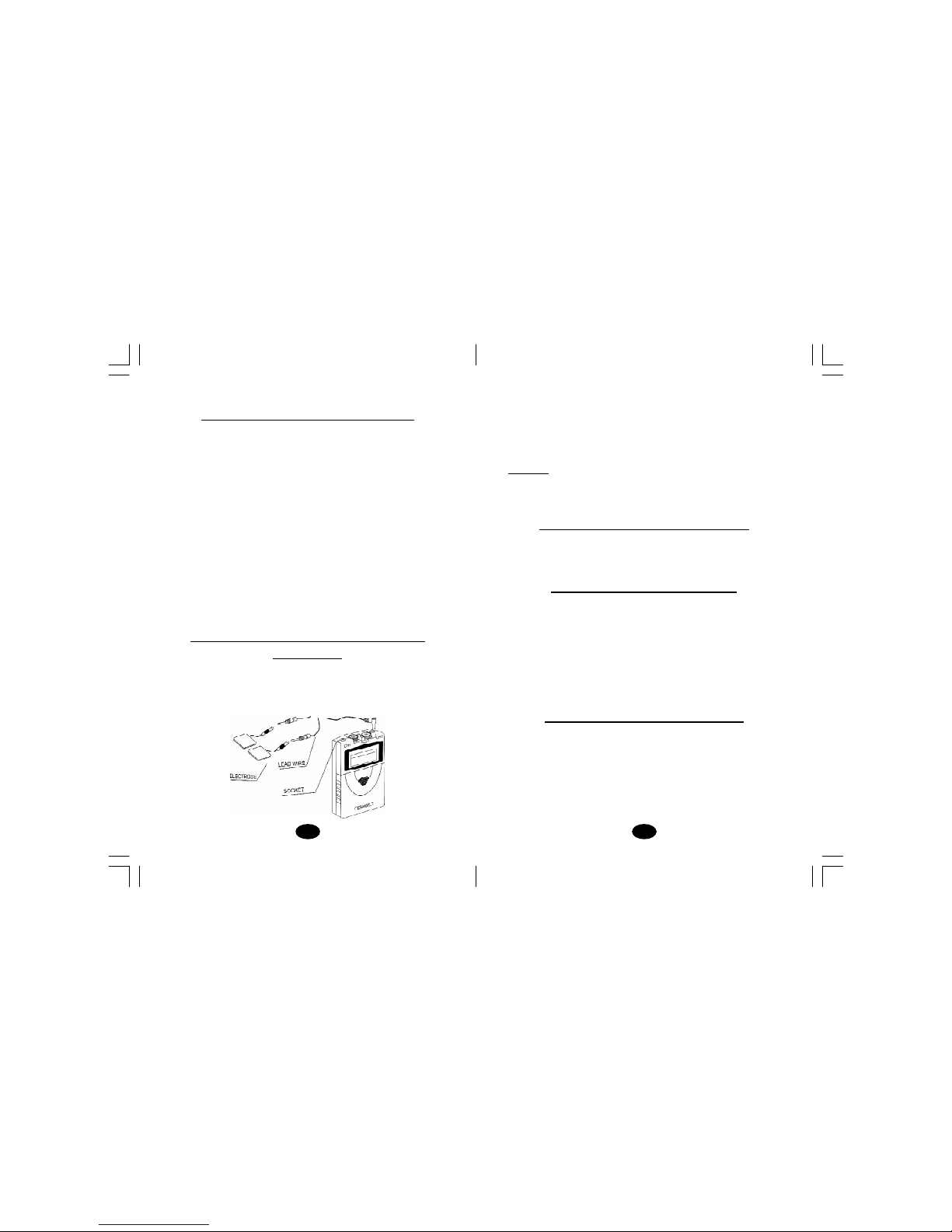
9. Powered muscle stimulators should be used only with the leads
and electrodes recommended for use by the manufacturer.
10.Portable powered muscle stimulators should not be used while
driving, operating machinery, or during any activity in which
involuntary muscle contractions may put the user at undue
risk of injury.