Contents
Important information
1About this document .. . . . . . . . . . . . . 3
1.1 Warnings and symbols .. . . . . . . 3
1.2 Copyright information .. . . . . . . . 4
2 Safety .. . . . . . . . . . . . . . . . . . . . . . . . . 4
2.1 Intended purpose .. . . . . . . . . . . 4
2.2 Indications .. . . . . . . . . . . . . . . . . 4
2.3 Contraindications .. . . . . . . . . . . . 4
2.4 Intended use .. . . . . . . . . . . . . . . 4
2.5 Improper use .. . . . . . . . . . . . . . . 4
2.6 General safety information .. . . . . 5
2.7 Systems, connection with other
devices .. . . . . . . . . . . . . . . . . . . 5
2.8 Standards .. . . . . . . . . . . . . . . . . 5
2.9 Specialist personnel .. . . . . . . . . 5
2.10 Electrical safety .. . . . . . . . . . . . . 6
2.11 Essential performance charac-
teristics .. . . . . . . . . . . . . . . . . . . 6
2.12 Notification requirement of seri-
ous incidents .. . . . . . . . . . . . . . . 6
2.13 Only use original parts .. . . . . . . . 6
2.14 Transport and packaging .. . . . . . 6
2.15 Disposal .. . . . . . . . . . . . . . . . . . 6
2.16 Protection from threats from the
Internet .. . . . . . . . . . . . . . . . . . . 6
Product description
3Overview .. . . . . . . . . . . . . . . . . . . . . . . 8
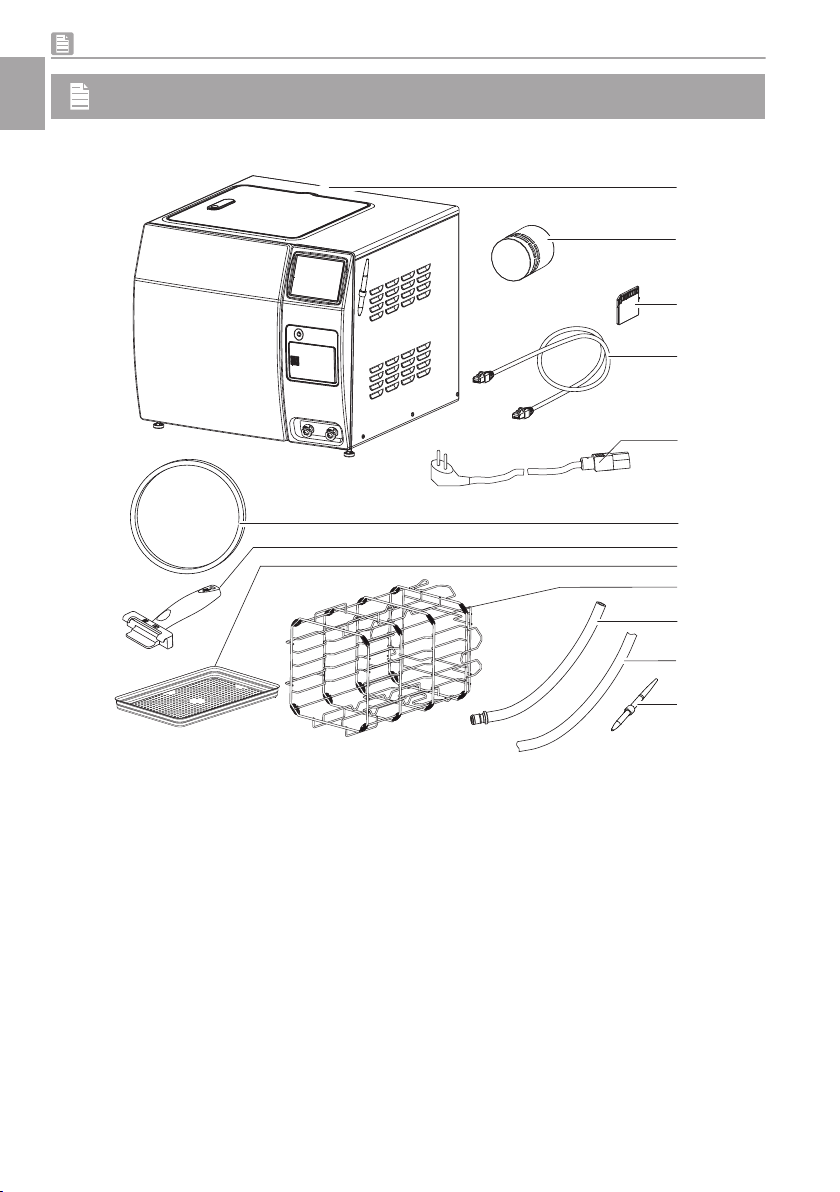
3.1 Scope of delivery .. . . . . . . . . . . . 9
3.2 Accessories .. . . . . . . . . . . . . . . . 9
3.3 Optional items .. . . . . . . . . . . . . . 9
3.4 Consumables .. . . . . . . . . . . . . . 9
3.5 Wear parts and replacement
parts .. . . . . . . . . . . . . . . . . . . . . 9
4Technical data .. . . . . . . . . . . . . . . . . . . 10
4.1 Type plate .. . . . . . . . . . . . . . . . . 12
4.2 Evaluation of conformity .. . . . . . 12
5 Operation .. . . . . . . . . . . . . . . . . . . . . . 13
5.1 Hygoclave 90 .. . . . . . . . . . . . . . 13
5.2 Safety devices .. . . . . . . . . . . . . . 15
5.3 Overview of programs .. . . . . . . . 17
Assembly
6 Requirements .. . . . . . . . . . . . . . . . . . . 18
6.1 Installation/setup room .. . . . . . . 18
7 Installation .. . . . . . . . . . . . . . . . . . . . . . 18
7.1 Carrying the unit .. . . . . . . . . . . . 18
7.2 Remove the transport locks .. . . 18
7.3 Setting up the unit .. . . . . . . . . . . 18
7.4 Removing the protective film
from the touch screen .. . . . . . . . 19
7.5 Checking the SD memory card .. 19
7.6 Checking the air filter .. . . . . . . . . 20
7.7 Connecting the unit .. . . . . . . . . . 20
7.8 Connecting the device to the
network .. . . . . . . . . . . . . . . . . . . 22
7.9 Connecting a label printer .. . . . . 22
8Configuring the unit .. . . . . . . . . . . . . . 24
8.1 Selecting the access level .. . . . . 24
8.2 Entering dealer information .. . . . 24
8.3 Language selection .. . . . . . . . . . 24
8.4 Configuring the device with a
network connection .. . . . . . . . . . 24
8.5 Set the date and time. .. . . . . . . . 24
8.6 Parameter selection .. . . . . . . . . . 25
8.7 Configuring a log printer .. . . . . . 25
8.8 Configuring the label printer .. . . . 25
8.9 Configuring the network drive .. . 25
8.10 Monitoring the unit via the net-
work .. . . . . . . . . . . . . . . . . . . . . 26
8.11 User management .. . . . . . . . . . . 27
8.12 Setting up the calendar .. . . . . . . 27
8.13 Touch screen .. . . . . . . . . . . . . . . 27
8.14 Resetting counter readings .. . . . 28
9Test programs .. . . . . . . . . . . . . . . . . . . 28
9.1 Vacuum test .. . . . . . . . . . . . . . . 28
9.2 Bowie-Dick test .. . . . . . . . . . . . . 29
10 Validation for commissioning .. . . . . . . 29
Contents
6046100064L02 2007V003 1
EN