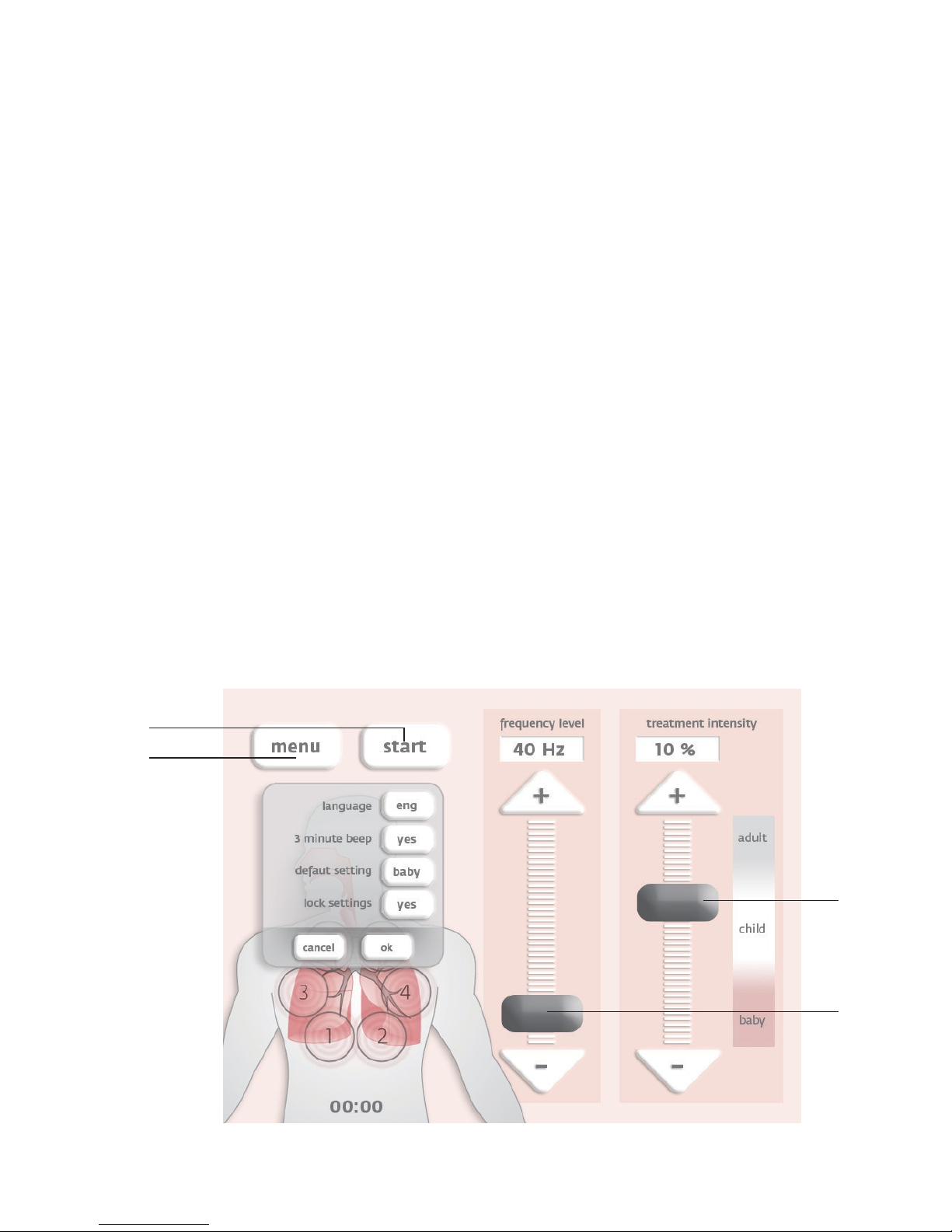
[1] Indications for use
The Frequencer® V2x provides airway clearance therapy and
promotes
bronchial drainage by inducing
vibration
in the chest walls. This device
is intended to be a
component
of chest physiotherapy by providing a
convenient method of external thorax
manipulation.
It
is intended for patients having respiratory ailments which
involve
defective mucociliary clearance, as is typical in patients suffering
from
cystic fibrosis as well as chronic
bronchitis,
bronchiectasis,
ciliary
dyskinesia syndromes, asthma, muscular dystrophy,
neuromuscular
degenerative disorders,
post-operative
atelectasis and thoracic
wall
defects. Indications for this form of therapy are described in the
Clinical
Practice Guidelines for Postural Drainage Therapy of the
American
Association for Respiratory
Care (AARC)
published in 1991
.
This
particular
device
provides a gentler,
less
painful form of therapy from the
traditional
“clapping” method of postural drainage therapy,
allowing it
to be used
on patients who cannot be treated by
clapping.
The Frequencer® V2x is suitable for use in all patient-care
environments
including
home health-care.
WARNINGS
•Use only original Frequencer® V2x parts or replacement parts sold
by Dymedso. Do not substitute the power supply.
•If patient experiences any
discomfort
or pain while
using
the
Frequencer® V2x, stop the
treatment
and consult a
physician
.
•This instruction manual is not intended to supersede established
medical protocols.
•This device is not a form of life support.
•Repairs must be performed by the manufacturer, the
manufacturer’s distributor or authorized agent. Do not open this
equipment including the transducer or the power supply.
•This equipment is not suitable for use in the presence of a
flammable anaesthetic mixture in combination with oxygen or air, or
in the presence of nitrous oxide.
•To avoid electrical shock, disconnect the power cord before
cleaning. Do not immerse the device in any fluids.
The Frequencer® instruction manual