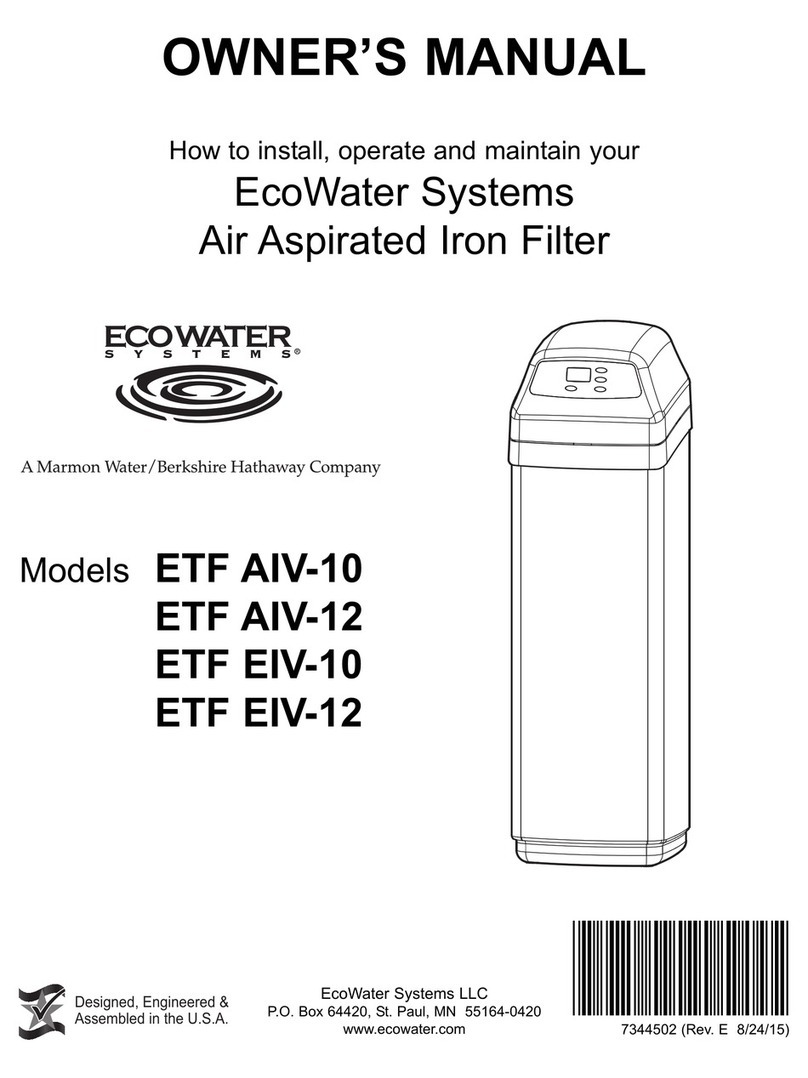
4
ECOWATER
S Y S T E M S
Water, and Water Conditioning
WATER
Man’ very exi tence depend on water. It i 1 of the
ba ic commoditie of life. Water i be t a nature
provide it, i a common mi conception. Practically
all natural water need refinement or treatment to
make it afe to drink or more ati factory to u e.
The earth’ water upply cycle tart in the upper
cloud layer . A it fall to the earth a rain or now,
it pick up impuritie and ga e from the atmo-
phere. Landing on earth, it eep over and through
the ground, di olving earth mineral . Pa ing
through lime tone, it di olve calcium and magne-
ium, the hardne mineral . Iron depo it impart
iron to the water. Acidity and ediment are other
water condition .
Municipal water upplie come from urface re er-
voir , uch a lake and river , or from underground
re ervoir . U ually, municipalitie chlorinate the wa-
ter to make it afe to drink. Sediment i removed by
filtration. Ta te and odor are reduced or elimi-
nated. The water i conditioned to comply with cer-
tain pecification . However, hardne mineral ,
ta te and odor are not alway reduced to the mo t
de irable level .
Underground re ervoir provide our private water
upplie . Becau e the water i raw and untreated, it
can have varying amount of hardne , iron, ta te ,
odor , acidity, or combination of the e. Different lo-
calitie and water level affect mineral content.
WATER CONDITIONING
Water conditioning i the treatment of four general
condition . The e are: (1) Hardness, (2) Iron, (3)
Acidity, (4) Sediments.
(1) HARDNESS i a term to de cribe the pre ence
of calcium and magne ium mineral in water. A
chemical analy i accurately mea ure the amount
of mineral in grain weight. For example, 1 gallon of
water with 5 grain per gallon (gpg) hardne ha
di olved mineral , that if olidified, about equal
the ize of 1 ordinary a pirin tablet. One gallon of wa-
ter, 25 gpg hard, ha a mineral content equal in ize
to 5 a pirin tablet . Water hardne varie greatly
acro the country. It generally contain from 3 to
100 gpg.
Hard water affect living in general. Hardne miner-
al combine with oap to make a oap curd. The
curd greatly reduce the cleaning action of oap.
Precipitated hardne mineral form a cru t on
cooking uten il , appliance , and plumbing fixture .
Even the ta te of food are affected. A water oft-
ener remove the hardne mineral to eliminate
the e problem , and other . Page 16--17 de cribe
how the EcoWater conditioner work .
Sodium Information: Water oftener u ing odium
chloride ( alt) for regeneration add odium to the
water. Per on on odium re tricted diet hould
con ider the added odium a part of their overall in-
take.
(2) IRON in water i mea ured in part per million
(ppm). The total* ppm of iron, and type or type *, i
determined by chemical analy i . Four different
type of iron in water are: ❶Ferrou (clear water),
❷Ferric (red water), ❸Bacterial and organically
bound iron, ❹Colloidal and inorganically bound iron
(ferrou or ferric).
*Water may contain one or more of the four type of
iron and any combination of the e. Total iron i the
um of the content .
❶Ferrou (clear water) iron i oluble and di olve
in water. It i u ually detected by taking a ample of
water in a clear bottle or gla . Immediately after tak-
ing, the ample i clear. A the water ample tand ,
it gradually cloud and turn lightly yellow or brown
a air oxidize the iron. Thi u ually occur in 15 to
30 minute . An EcoWater conditioner will remove
moderate amount of thi type of iron*.
*Capacity to remove clear water iron wa te ted in
the field by the manufacturer.
❷Ferric (red water), and ❸Bacterial and organically
bound iron are in oluble. Thi iron i vi ible immedi-
ately when drawn from a faucet becau e it ha oxi-
dized before reaching the home. It appear a mall
cloudy yellow, orange, or reddi h u pended par-
ticle . After the water tand for a period of time, the
particle ettle to the bottom of the container. Gener-
ally the e iron are removed from water by filtration.
Chlorination i al o recommended for bacterial iron.
An EcoWater conditioner will remove minimal quan-
titie ( ee pecification ) of ferric iron.
continued