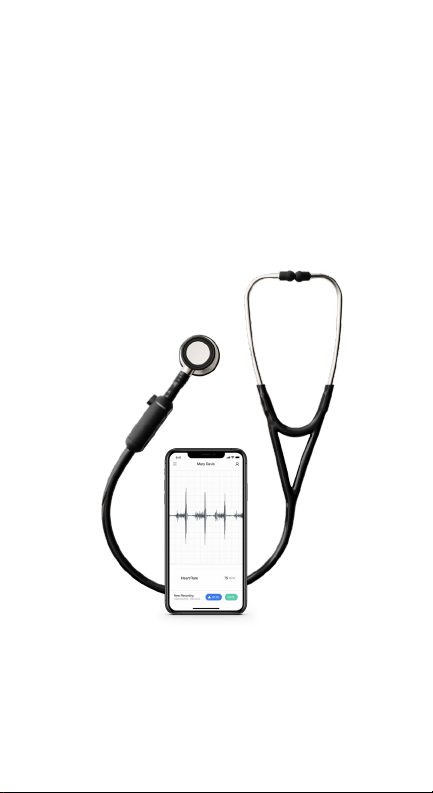
Eko Devices, Inc. Eko CORE User manual

© 2020 Eko Devices, Inc.Model 2nd Generation
User Manual
2
1. Indications for Use
The Eko CORE is an electronic stethoscope that enables
amplification, filtering, and transmission of auscultation sound
data (heart, lungs, bowel, arteries, and veins), whereby a clinician
at one location on network can listen to the auscultation sounds
of a patient on site or at a different location on the network. Eko
CORE is intended for use on pediatric and adult patients. The Eko
CORE is intended to be used by professional users in a clinical
environment or by lay users in a nonclinical environment. The
device is not intended for self-diagnosis.
Figure 1
Fully assembled digital stethoscope and mobile app

3
2. Introduction
The CORE is designed to support healthcare professionals in
listening to sounds produced by the body, primarily lung, heart,
and bowel sounds. CORE also enables regular users to record,
store and share their body sounds with their physician. CORE
includes a device that is attached to a stethoscope (CORE
attachment) and an application, the Eko App.
CORE features sound amplification and audio transmission to
a smartphone via Bluetooth that allows the user to open and
playback sounds in a mobile application on compatible iOS and
Android smartphones and tablets. The App provides the ability
for clinicians to save sounds within select Electronic Health
Record (EHR) systems, share recordings with other clinicians, and
annotate notes on recorded audio.
3. For Help and Assistance
Please contact Eko if you need assistance
or any product related concerns.
For more information please visit:
https://www.ekohealth.com/getstarted
Phone Support: 1.844.356.3384
This User Manual also applies to:
3M™ Littmann® CORE Digital Stethoscope
4
4. Equipment Symbols
REF
93%
15%
40°C
-30°C
0537
Instructions for use
European technical conformity
European Authorized Representative
Do not dispose with household waste
Emits Radio Frequency signal
Model number
Humidity range
Temperature range
Wireless Bluetooth communication
Manufacturer
Manufacturing date
Quantity
IP22 indicates protection against access to
hazardous parts with a finger, solid objects ≥ 12.5
mm diameter, and vertically falling water drops when
enclosure tilted up to 15 degrees.
MR Unsafe
IP22
MRMR

5
5. Cautions
To reduce the risk of device interference, keep CORE at least 1
meter away from all RF emitters including Wifi routers and radios.
Follow all cleaning and disinfecting instructions included in
this manual. Establish and follow a cleaning and disinfecting
schedule.
To reduce the risks associated with inaccurate data acquisition
store and operate this stethoscope only as instructed in this
manual. It is highly recommended that the battery be recharged
within thirty minutes of the LED indicator turning orange.
Recharge the battery using only the provided USB power cord
with a UL-certified USB wall charger (not provided).
DO NOT immerse the stethoscope in a liquid or subject it to any
sterilization processes other than those described in this manual.
To reduce the risks associated with very strong
electromagnetic fields avoid using the stethoscope near strong
radio frequency (RF) signals or portable and/or mobile RF
devices and/or specific RF emitters that are known sources of
electromagnetic disturbance such as diathermy, electrocautery,
RFID, security systems (e.g., electromagnetic anti-theft systems,
and metal detectors). Interference from hidden RF emitters like
RFID might cause packet loss and this will be visible as a “Poor
Bluetooth Signal” message on the mobile application. Move away
from the hidden RF emitter if this happens.
If sudden or unexpected sounds are heard, move away from any
radio transmitting antennas. Using accessories, transducers, and
cables not produced by Eko Devices, Inc. may result in increased
RF emissions or decreased immunity of the CORE.
Please read, understand, and follow all safety information
contained in these instructions prior to using the CORE. It is
recommended that these instructions be retained for future
reference.
To reduce the risk associated with an electrical shock do not
use the stethoscope without the analog stethoscope’s chest
piece in place.

6
CORE contains a Bluetooth wireless data link. The maximum
radio frequency field strength generated by the stethoscope
is below three volts per meter, a level that is considered safe
to use with other medical devices. However, audio, video, and
other similar equipment may cause electromagnetic interference.
If such devices are encountered and cause interference,
immediately move CORE away from that device and/or turn the
Bluetooth feature OFF.
Consult with your physicians when using the Eko device.
To ensure high quality sounds location and position of CORE
placement should be taken into consideration when auscultating.
To improve Bluetooth connection, reduce the distance and/or
allow a line of sight between Eko device and mobile device. The
Bluetooth range will be reduced when objects (walls, furniture,
people, etc) are between the Eko device and a paired mobile
device.
To reduce risk of asphyxiation and strangulation, ensure that
all components are properly attached and stored. Keep away
from children.

7
6. EMC Compliance
FCC Intentional Radiator Certification
Contains FCC ID: 2ANB3-E6
Contains IC: 23063-E6
47 CFR Part 15.105 required statement for Class B:
This equipment has been tested and found to comply with the
limits for a Class B digital device, pursuant to part 15 of the FCC
Rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency
energy and, if not installed and used in accordance with
the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that
interference will not occur in a particular installation.
If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the
equipment off and on, the user is encouraged to try to correct
the interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different
from that to which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician
for help.

8
Canada regulatory statement(s):
This device complies with Industry Canada license-exempt RSS
standard(s). Operation is subject to the following two conditions:
(1) This device may not cause interference; and (2) This device
must accept any interference, including interference that may
cause undesired operation of the device.
Le présent appareil est conforme aux CNR d’Industrie Canada
applicables aux appareils radio exempts de licence. L’exploitation
est autorisée aux deux conditions suivantes : (1) l’appareil ne
doit pas produire de brouillage, et (2) l’utilisateur de l’appareil
doit accepter tout brouillage radioélectrique subi, même si le
brouillage est susceptible d’en compromettre le fonctionnement.
NO MODIFICATION
Modifications to this device shall not be made without the
written consent of Eko Devices, Inc. Unauthorized modifications
may void the authority granted under Federal Communications
Commission rules permitting the operation of this device.
EMC Compliance Europe
This equipment complies with the EMC requirements of the IEC
60601-1-2.

9
7. Contents and Operation
CORE device includes (1) CORE attachment, (2) tubing adapters,
and (1) micro USB cable and the Eko App. The compatible
hardware and software platforms are listed below.
Compatible Stethoscopes
CORE is designed and tested to be compatible with the
3M™ Littmann® Cardiology III™, 3M™ Littmann® Cardiology
IV™, WelchAllyn Harvey™ Elite®, Medline and ADC analog
stethoscopes. CORE is compatible with many other stethoscope
brands and models, but there are no performance guarantees
when using other stethoscope brands or models.
NOTE: CORE is not compatible with Sprague stethoscopes or
other digital stethoscopes.
Bluetooth and Data Connection
In order to transmit sounds to the Eko App, the stethoscope and
device must be connected via Bluetooth, and in order to fully use
certain functions, the mobile device must be connected to the
internet via cellular data connection or Wi-Fi. Please keep CORE
and Eko App within 15 feet for optimum Bluetooth connection. In
the highly unlikely condition that the device is rebooted, revert to
using the analog mode. The digital mode should restart in less
than ten seconds.

10
System Requirements
The mobile app software can be used on iPhone 5S, iPhone 6/6
Plus, iPhone 6s/6s Plus, iPhone 7/ 7 Plus, iPhone 8/8 Plus, iPhone
X, XS, XS Max, iPad* Mini 2/3/4, iPad Air/Air 2, iPad Pro, iPod
Touch 6G, and iPad 5th and 6th generations with iOS 12.0 and
higher. The mobile app software can also be used with Android
devices with BLE support (Bluetooth 4.0) and Android 8.0 and
above.
CORE uses Bluetooth Smart; mobile devices used must be
compatible with Bluetooth Smart.
*iPhone, iPad, iTunes, and iOS are registered trademarks of
Apple, Inc.
*Bluetooth is a registered trademark of Bluetooth SIG, Inc.
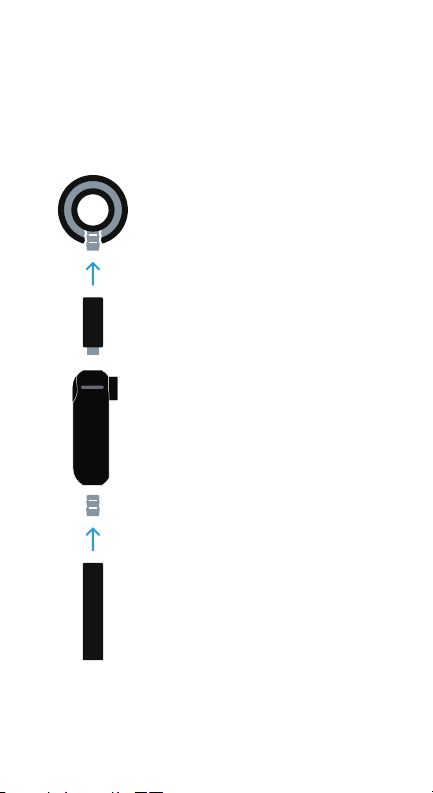
11
8. Installation to Existing Stethoscopes
This section is not required for pre-assembled digital stethoscopes
Step One
Grip chest piece with one hand and pull the
tubing with force using the other hand to
detach the chest piece from the tubing of
the existing stethoscope. Insert the chest
piece into the Eko-compatible adapter
tubing provided
Step Three
Attach the tubing of the existing digital
stethoscope to the other end of the CORE
Attachment and assembly of the CORE
digital stethoscope is now complete
Step Two
Attach the CORE Digital Attachment to the
other end of the Eko-compatible adapter
tubing provided
Figure 2

12
9. CORE Use
Charge Battery
The battery in CORE will need to be charged; insert the included
micro USB cable into the USB port on the device and plug the
other end into a UL-certified USB wall charger. The LED will turn
solid yellow, signifying that it is charging. The LED will change to
solid green when the device is fully charged. The fully charged
battery should last for at least 8 hours in continuous transmission
mode (ON, Bluetooth paired with Eko App).
NOTE: CORE will not turn on while it is plugged in and charging.
Power Off
When CORE is turned Off, analog rather than digital sounds will
be transmitted and heard from the stethoscope. “OFF” is when
the toggle is protruding from the surface of the volume buttons.
Power On
Depress the power slider to move the switch from the OFF to the
ON position. “ON” is when the toggle is flush with the surface of
the volume buttons.
Test the Volume Level
CORE’s sound level can be amplified in 7 increments up to 40X
amplification of an acoustic stethoscope. Change the volume
level by clicking the plus (+) and minus (-) volume buttons on the
side of CORE.
Bluetooth Pairing
First, enable Bluetooth on the selected mobile device. On the
iOS device go to Settings > Bluetooth > and tap the slider to turn
Bluetooth ON.
The mobile device is now ready to record sounds from CORE. If
Bluetooth pairing is unsuccessful, an error message will appear
in the App and no sounds will be recorded. If the Bluetooth
connection is successful the LED will turn from flashing white to
solid white (See Section 6.1 for the LED states of the device).

13
Setting up a PIN
Create a secure 4-digit PIN by logging in to the mobile
application. Navigate to the Menu screen by selecting the icon
on the top left of the Mobile App home screen.
Next, select Account Settings > Create Pin. Follow the
instructions on the screen to create and save a 4 -digit PIN. You
will need to enter your PIN twice for verification purposes.
Adding Notes to Recordings on Mobile App
To create notes on any patient recordings, log into the mobile
application. Access the list of patients by selecting the patients
tab on the top right of the home screen. Select the desired
patient and select a recording to add notes to.
On the bottom of the recording screen, select the Notes icon.
The Notes icon looks like a Post-It® with writing on it. Select “Add
Note” and begin typing your note. Select the check mark to save.
Operating the CORE
When using the CORE to assess and record heart sounds, it is
best to place the CORE stethoscope at the standard auscultation
points on the anterior chest wall as shown below with BLACK
dots (refer to Figure 4a).
When using the CORE to assess and record lung sounds, it is
best to place the CORE stethoscope at the standard auscultation
points on the anterior chest wall as shown below with BOTH
black and blue dots (refer to Figure 4).
The diaphragm side of the stethoscope should be placed on
user’s chest wall to assess for both heart and lung sounds. Only
use the bell (or closed bell) of the stethoscope when assessing
low frequency sounds as recommended by a clinician (refer to
Figure 2).
diaphragm
opened
bell
closed bell
Figure 3

14
Headset alignment
Before placing the eartips in your ears, hold the headset in front
of you with the eartubes pointing away. Once the eartips are in
your ears, they should point forward.
Open the diaphragm
When using a double-sided stethoscope (refer to Figure 3), you
need to open (or index) the bell or diaphragm by rotating the
chestpiece. If the diaphragm is open, the bell will be closed,
preventing sound from coming through the bell, and vice versa.
Figure 4a
Figure 4b

15
10. Cleaning
Cleaning and Disinfecting Procedure
The stethoscope and CORE should be disinfected between each
use. Infection control guidelines from the Centers for Disease
Control and Prevention (CDC) state that reusable medical
equipment, such as stethoscopes, must undergo disinfection
between patients. Standard stethoscope hygiene practices apply
to the Eko device.
All external parts of the hardware should be disinfected with
70% isopropyl alcohol wipes. Under normal conditions, it is not
necessary to remove CORE attachment from the stethoscope
tubing during the disinfecting procedure.
NOTE: DO NOT immerse the device in any liquid or subject it to
any high-pressure/autoclave sterilization processes.
If it becomes necessary to remove CORE, pull the stethoscope
tubing off of the metal stem of the CORE attachment on both
ends. Wipe all parts of the stethoscope clean with 70% isopropyl
alcohol wipes or disposable wipe with soap and water including
CORE’s surface, stethoscope tubing, tubing connector, and
chest piece. A 2% bleach solution may be used to disinfect your
stethoscope tubing, tubing connector, and chest piece; however,
the tubing may become discolored after exposure to bleach.
To prevent staining of stethoscope tubing, avoid contact with
pens, markers, newsprint, or other printed material. It is good
practice to wear your stethscope over a collar whenever
possible.
Reassemble the stethoscope by reinserting the metal stems of
the CORE attachment into the stethoscope tubing as described
above in the installation section.

16
11. Operating Conditions
Environmental
The operating temperature range of CORE is -30° to 40°C (-22°
to 104°F), and 15% to 93% relative humidity.
The storage and transport range is -40° to 55°C (-40° to 131° F),
and 15% to 93% relative humidity. Acceptable pressure is 1 atm.
Avoid exposure to extreme heat, cold, solvents and oils. Extreme
heats and colds will negatively affect the lithium ion battery in the
device and may affect battery life.
No Modifications
Failure to follow care and maintenance recommendations could
result in damage to the internal components of CORE. Internal
damage to the product could cause malfunction of the product,
which may lead to complete loss of function. If problems are
encountered with CORE, do not attempt to repair it. Please notify
our support team for assistance.
Disposal
If the enclosure of the Eko device is damaged, please dispose of
it appropriately.
12. Warranty
Eko provides a limited warranty for CORE. Please visit ekohealth.
com/warranty for a full description of the warranty.
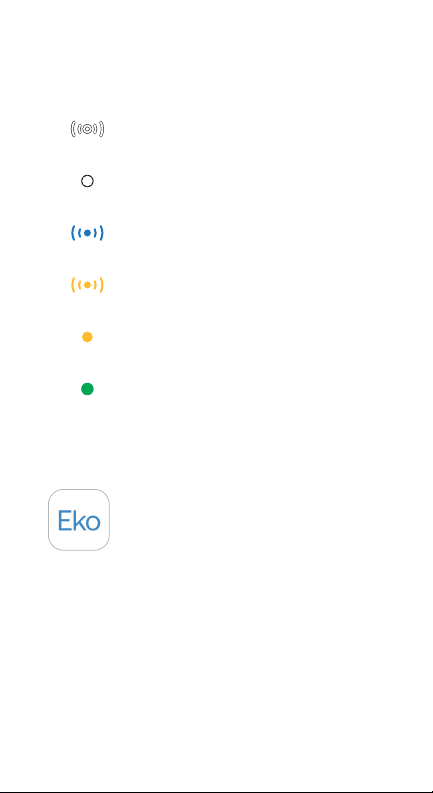
17
13. CORE Modes and
Corresponding LED States.
14. Eko App
Download the Eko app, available on the App Store® and Google
Play and follow the on-screen instructions to connect to CORE
(as shown on the next two pages).
Bluetooth must be enabled in the mobile or desktop’s Bluetooth
settings in order to use CORE with the Eko App.
When using the Eko Dashboard and Eko App, enable device
and networking security features to protect patient data that is
created and stored using this software, in addition to security
features embedded in the system. Update to the latest version
of the Eko App.
CORE is on & seeking device
CORE is on & connected
CORE is recording
CORE is off & charging
CORE is low on battery
CORE is fully charged
(Blinking)
(Blinking)
(Blinking)
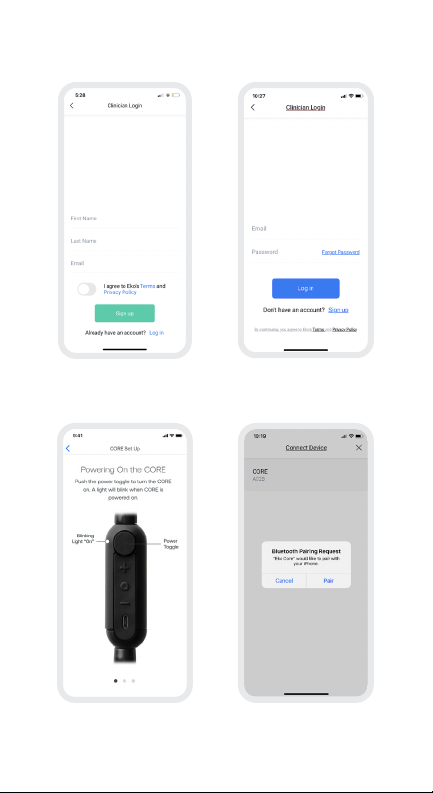
18
14a. Eko App – Provider Workflow
➊
Sign up:
Create your Eko account by entering in name
and email address
➌
Turn on CORE
➋
Login:
Enter in your login credentials
➍
Pair CORE
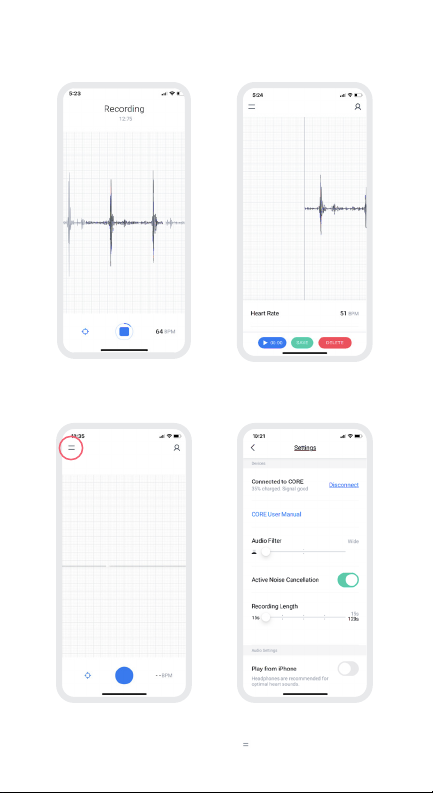
19
14a. Eko App – Provider Workflow
➎
Start Recording:
Place CORE on the patient’s chest; Press the
blue button to start recording.
➐
Eko Settings Menu
Adjust your settings by clicking on the ( ) top left home screen
➏
Save Recording:
Click save once your recording is complete

20
14b. Eko App – Patient Workflow
➊
Sign up
➌
Turn on CORE
➋
Setup
➍
Pair CORE
Table of contents
Other Eko Devices, Inc. Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Contec Medical Systems Co.
Contec Medical Systems Co. 22-01-CMS50D instructions

Care Fusion
Care Fusion Infant Flow Comprehensive Quick Guide

Mastercare
Mastercare Silk Master MC 001-1839 user guide

Bionet
Bionet Cardio 7 Service manual

ARJO HUNTLEIGH
ARJO HUNTLEIGH First Step Select user manual
New Age
New Age iONECARE user manual

B. Braun
B. Braun Aesculap Spine Instructions for use/Technical description
B-K Medical
B-K Medical bk5000 Setup guide

PRISM+
PRISM+ CP user manual

ARJO HUNTLEIGH
ARJO HUNTLEIGH Akron Streamline 2 Section Instructions for use

B. Braun
B. Braun Aesculap 012978 Instructions for use/Technical description
Hillrom
Hillrom Advanta P1600 Service manual