764000-00154 Rev 2 EN
•Avoid exposure to extreme hot or cold temperatures outside the range of 0°C to 38°C (32°F
to 100°F). Exposure to such conditions may cause the device to malfunction
•Do not attempt to replace the device battery. If the device is not working, contact Customer
Service
•Do not open or take apart the case or attempt to repair or modify the device. There are no
user-serviceable parts. If the device is not working, contact Customer Service
•As the device contains a lithium-ion battery, do not intentionally damage, burn or puncture
the device
•Wireless communications equipment, such as wireless home network devices, mobile
phones, cordless telephones and their base stations, and walkie-talkies, can affect this
equipment. Keep gammaCore at least 3.3 m (10.8 ft) away from these items while in use
If the device is not working, contact Customer Service (refer to Section 24).
6. WILL I STILL NEED TO TAKE MEDICATIONS?
You and your healthcare provider (HCP) should discuss your ongoing treatment routine, including the
use of any additional therapies and/or medications. It is important to always follow your HCP’s
recommendations about your medications. gammaCore can be used with existing medications as there
have been no reported device-drug interactions.
7. ADDITIONAL INFORMATION FOR HEALTHCARE PROVIDERS
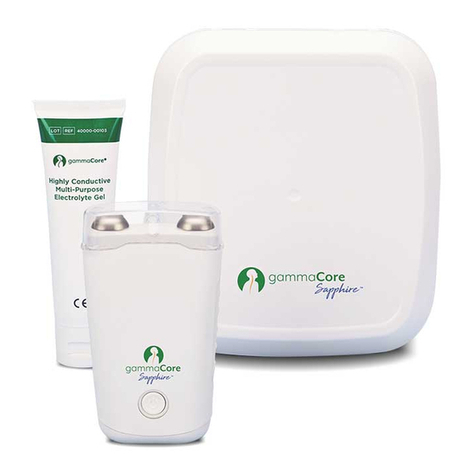
gammaCore Sapphire™ (non-invasive vagus nerve stimulator) is a hand-held, rechargeable, portable
device consisting of a rechargeable battery, signal-generating and -amplifying electronics, and a control
button for the patient to control the signal amplitude. It is intended for single patient use and can deliver
multiple stimulations a day. The device provides visible (display) and audible (beep) feedback on the
device and stimulation status. A pair of stainless-steel surfaces, which are the skin contact surfaces
(“stimulation surfaces”), allow the delivery of a proprietary electrical signal. The patient applies
electroCore-approved gel to the stimulation surfaces to maintain an uninterrupted conductive path from
the stimulation surfaces to the skin on the neck. Tubes of electroCore-approved gel are provided with
each unit and refill kit for this purpose. The stimulation surfaces are capped when not in use.
gammaCore produces a low-voltage electric signal consisting of five 5,000-Hz pulses that are repeated at
a rate of 25 Hz. The waveform of the gammaCore pulse is approximately a sine wave with a peak voltage
limited to 24 Volts when placed on the skin and a maximum output current of 60mA.
The HCP must brief the patient on the following items:
•The HCP must inform the patient using gammaCore to notify them of any changes in health
status. The HCP must re-evaluate the patient’s suitability for treatment using gammaCore
based on the patient’s new health information.
•The HCP should train patients in the proper use of gammaCore, inform them of all potential
risks and complications of treatment, and provide accompanying device labeling.