15 64000-00120 Rev 3
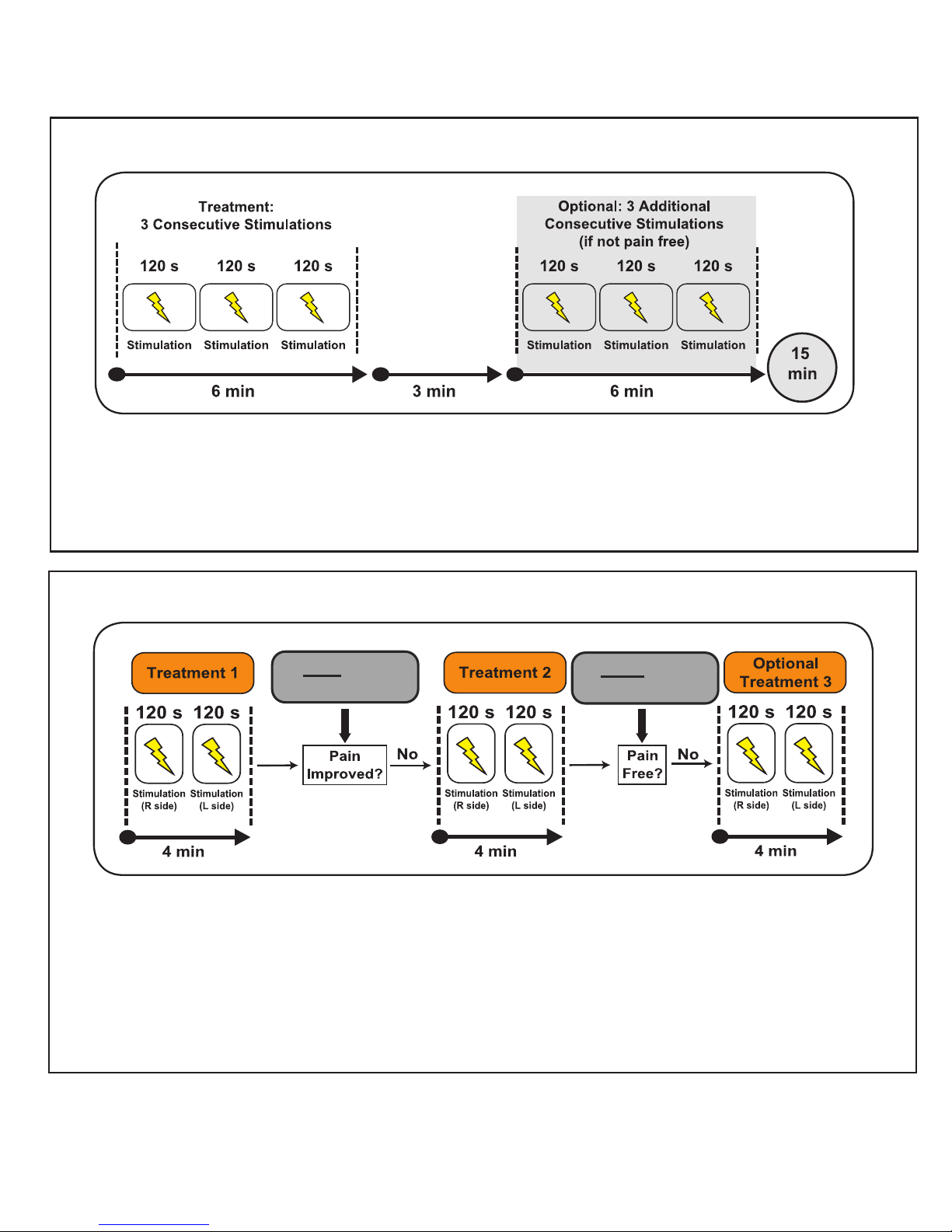
If the pain has not decreased twenty minutes
after the start of your rst treatment, you may
administer an additional treatment consisting of
two 2-minute stmulations (one on the right side of
the neck, one on the left side of the neck).
If you are not pain-free two hours after the start
of your rst treatment, you may administer a third
treatment consisting of two 2-minute stimulations
(one on the right side of the neck, one on the left
side of the neck).
Please see Figure 2 at the end of this section
for an example of the treatment of one migraine
headache.
NOTE: The length of each stimulation (120
seconds) provides a sufcient amount of time for
correct positioning of gammaCore and for setting
the appropriate stimulation intensity.
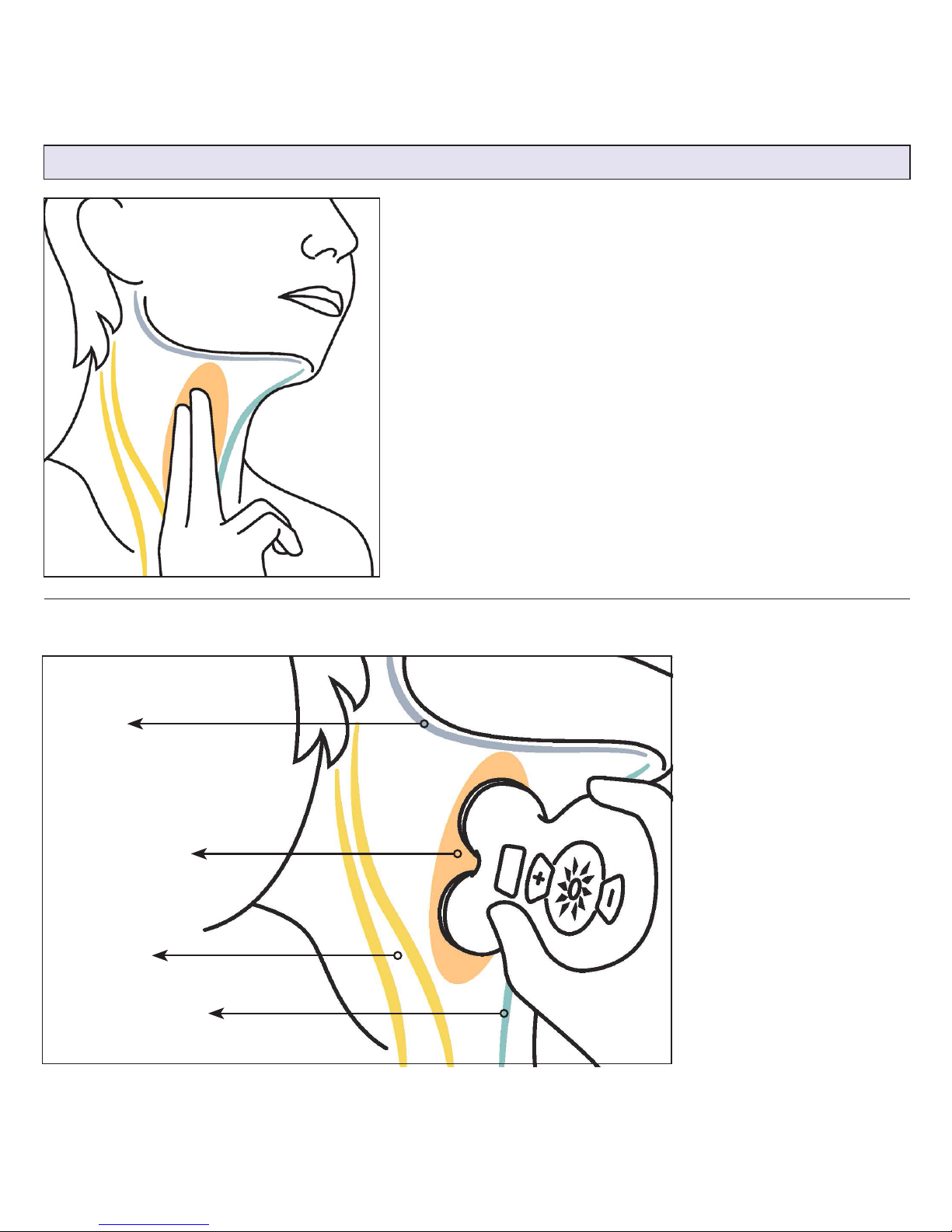
NOTE: Make sure that both stimulation surfaces
are in contact with the skin during the stimulation.
Checking in a mirror may help until you become
familiar with the device and its correct positioning.
10. After each stimulation, remove the device
and turn it off by pressing the (+) and (-)
buttons at the same time or wait for the
device to automatically turn off. After
completing the stimulation, the device will
display the number of stimulations and days
remaining and the last stimulation intensity
before automatically turning off.
NOTE: A stimulation stops automatically after 120
seconds. The device will make 2 short beeps and
automatically stop stimulation.
NOTE: The number of days and stimulations
remaining can be viewed by turning the device
on. However, do not turn the stimulation intensity
higher than three (3) until preparing for a stimula-
tion. The device counts each time the stimulation
intensity is higher than three (3). The device has
a limited number of stimulations.
NOTE: Some users who attempt multiple stimula-
tions in a row could experience an issue where the
device will not turn on immediately. gammaCore
has a delay feature to prevent accidental starts
due to an unintentional button press. To resolve,
wait 10 seconds before turning the device on
again and repeat steps 5-9a for the acute treatment
of episodic cluster headache and steps 5-9b for
the acute treatment of migraine headache.
NOTE: If you have trouble turning on the device,
press the (+) and (-) buttons at the same time for
10 seconds and then release. Wait 5 seconds and
press the top (+) button again to turn on the device.
11. Clean the device by wiping the leftover gel
off the stimulation surfaces with a soft dry
cloth (refer to Section 10).
12. Clean the excess gel off your neck with a
cloth or tissue. The gel is not intended to be
left on the skin and may cause skin irritation
for some people.
13. Put the caps back on the device after use.