ElectroCore TAC-STIM User manual
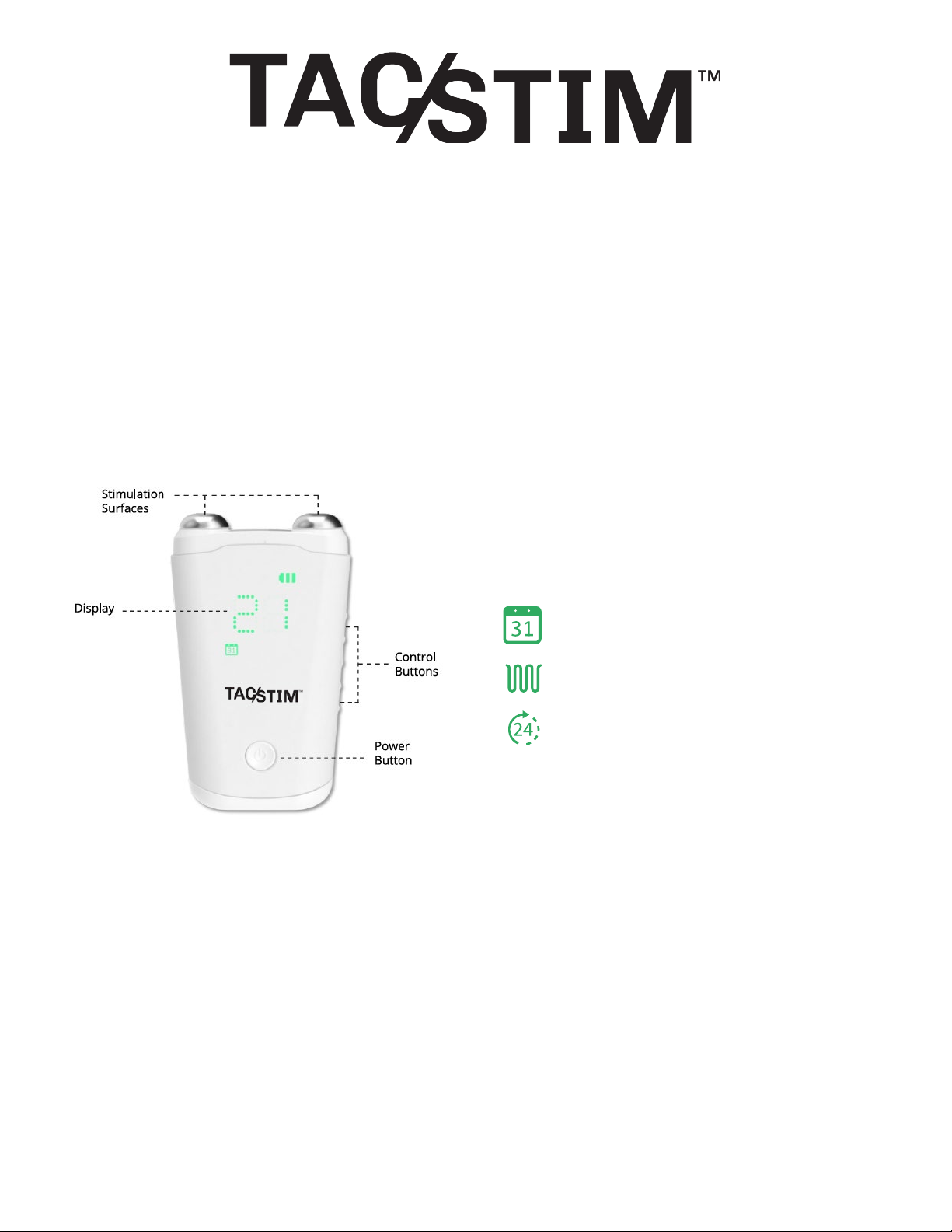
The icons below indicate what the
number on the display represents.
User Guide
About TAC-STIM
TAC-STIM™ transdermal vagus nerve stimulation (tVNS) enhances human performance by improving memory
retention, attention, and mood, accelerating training, and decreasing fatigue.
TAC-STIM tVNS delivers proprietary electrical signals via two small electrodes placed on the skin at the cervical
level. The electrical energy stimulates the vagus nerve, which triggers neurotransmitters in the brain.
NOTE: TAC-STIM is active for 36 months when turned on, and the session intensity increased past three.
Get to know TAC-STIM
Sessions remaining in a
24-hour period
Intensity level
Months remaining
Charging TAC-STIM
-Plug the charging cord into the back of the charging case and connect it to an electrical outlet.
-Place TAC-STIM into the charging case.
-“Ch” will appear on the display, indicating it is placed correctly and charging.
-Charge TAC-STIM for at least one hour prior to first use.
Cleaning TAC-STIM
-Clean TAC-STIM after each use by gently wiping the case and the stimulation surfaces with a soft, dry cloth to
remove leftover gel.
-Put the cap back on the product after use to protect the stimulation surfaces from dirt, debris, and damage.
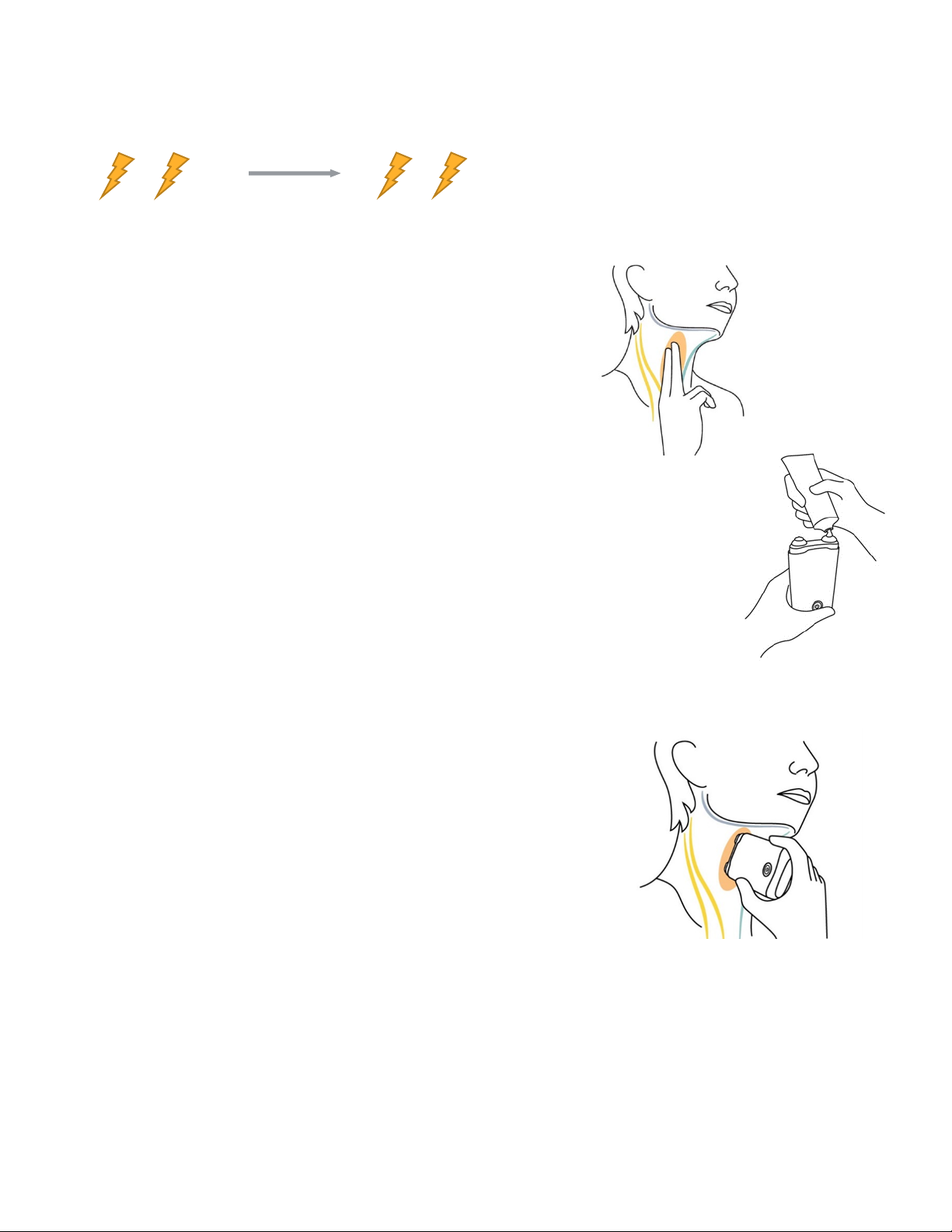
1. Locate your vagus nerve.
1. Use 2 fingers to locate your pulse in the cervical region;
the vagus nerve is in the same area.
2. Make sure the application site is clean and dry.
3. Remove anything blocking the application site.
3. Administer the sessions.
7. Place TAC-STIM over the vagus nerve at the cervical region.
8. Increase the intensity level until you notice a slight muscle
contraction at the corner of your mouth.
9. TAC-STIM will stop automatically after 2 minutes.
NOTE: You will likely feel muscle contractions at the application site and
may experience a lip pull. These are normal and should stop after the
session is complete. If muscle contractions are too strong or
uncomfortable, try:
• Lowering the intensity level by pressing the bottom area of the
control button.
• Repositioning the electrodes on the neck over the pulse and
slowly increasing the intensity level again by pressing the top
area of the control button.
If the intensity level is still too strong, stop the session and turn
TAC-STIM off by pressing and holding the power button for 3 seconds.
NOTE: Additional gel may be needed between sessions.
2. Prepare your TAC-STIM.
4. Remove the cap.
5. Apply a pea-sized amount of the provided gel to the stimulation surfaces.
a. Not applying the gel as described may cause the session to be
uncomfortable or less effective.
6. Turn TAC-STIM on by pressing the power button. When TAC-STIM is ready for
use, it will beep once.
Using TAC-STIM
Morning:
Evening:
26 additional sessions
available for use as
needed.
For more assistance, visit tac-stim.com or call 888-901-7846.
2 two-minute sessions
2 two-minute sessions

Warnings and Precautions
WARNINGS
Do not use TAC-STIM if:
-You have an active implantable medical device, such as a pacemaker, hearing aid implant, or any
implanted electronic product.
-You are using another product at the same time (e.g., TENS Unit, muscle stimulator).
-You are driving, operating machinery, or during any activity that may put patient at risk of injury.
-You are in the presence of strong electromagnetic fields, such as MRI scanners.
-You are in an explosive atmosphere or in the presence of flammable gas mixtures.
-You have an open wound, rash, infection, swelling, cut, sore, drug patch, or surgical scar(s) on the neck at
the application site.
-You have wet skin, are in the water, or just stepped out of the water (e.g., shower, bath, pool).
It is not recommended to use TAC-STIM:
-If you have had surgery to cut the vagus nerve at the neck as it may not be effective.
-If you are younger than 12.
-If you are pregnant or breastfeeding.
-More than 24 times a day.
PRECAUTIONS
-TAC-STIM should only be used as described in the User Guide.
-Only use a TAC-STIM-supplied conductive gel.
-Do not apply TAC-STIM across or through the head, directly on the eyes, covering the mouth, on the
chest, on the upper back, or over the heart.
-Do not use TAC-STIM if there are signs of damage or defects.
-Do not use if an error code is displayed on the screen when the product is turned on.
-Do not submerge TAC-STIM in water; it is not water resistant.
-Store in a safe location out of reach of children.
Potential Adverse Reactions
Users with sensitive skin may experience application site discomfort, irritation and/or redness. If you experience
light-headedness, dizziness, or chest pain, excessive skin irritation, local pain, face/head/neck area (including
toothache), muscle twitching, tingling, contractions, or other adverse reactions, DISCONTINUE USE. These reactions
typically resolve after the session is complete; however, if it persists after the session, consult your physician.
Troubleshooting
TAC-STIM does not turn on
• TAC-STIM is not charged. Charge TAC-STIM by placing it in the charging case.
• Restart TAC-STIM by pressing and holding the power button for 3 seconds.
TAC-STIM does not charge
• If “Un” is displayed, remove and place back in charging case. If “Un” continues to be displayed, move product
around in the charging case.
• Ensure the AC power cord is plugged into a live power outlet and into the charging case. Only use the provided
AC power cord with the charging case.
TAC-STIM displays an error code
• Contact the TAC-STIM Customer Experience Team.

Expiration date
Follow operating instructions
Lot number
Manufacturer
Catalog number / Reference number
IP22
Protection from solid foreign objects ≥12.5 mm
and ingress of water at 15°
Electric shock hazard
Type BF applied part
Serial number
Storage temperature
Non-sterile xxxdyyzzzz (package
label)
Date of Manufacture on package label, where “D”
is the year of manufacture, e.g. 254DDK1001
indicates the year of manufacture is 2023
WARNING
Failure to follow instructions may
result in serious injury or death to
the patient or user
Non-ionizing electromagnetic radiation
PRECAUTION
Failure to follow instructions may
result in damage to the equipment or
degradation in the quality of treatment
Magnetic resonance unsafe
Refer to instruction manual
Authorized representative
Information or additional
information available
Keep away from sunlight
Separate collection for waste of electrical
and electronic equipment
Do not use if package is damaged
Date of Manufacture
Medical device
TAC-STIM Disposal
Regulations require that the disposal of electrical and electronic equipment is handled in a
controlled manner. A product that may be contaminated after use or contain chemicals or
elements that may present hazards to people, or the environment must be disposed of in
accordance with the applicable government regulations. Contact the TAC-STIM Customer
Experience Team if you have questions about the appropriate disposal of this product.
NOTE: TAC-STIM contains lithium batteries that the user cannot remove.
NOTE: Please dispose of TAC-STIM like a cell phone or other electronic product. Check with your
local municipality to do this according to your local electronic waste regulations.
Symbols and Nomenclature Description

Electrical Classification
Electrical Classification (TAC-STIM Product)
-UL 60601-1 Class III; EN 60601-1 Internally Powered Equipment.
-Type BF Applied part.
-IP22 Protected against ingress of solid foreign objects ≥ 12.5 mm diameter and protected against
vertically falling water drops when enclosure tilted up to 15°.
-Product contains Bluetooth RF transmitter: Frequency range of 2.379 to 2.496 GHz, GFSK Modulation,
1mW max power.
Electrical Classification (Charging Case)
-UL 60601-1 Class III.
-Accessible Part.
-IP22 protected against ingress of solid foreign objects ≥ 12.5 mm diameter and protected against
vertically falling water drops when enclosure tilted up to 15°.
Electromagnetic Compatibility Guidance
All engineering specifications have passed the required test levels. All specifications are available upon request.
Handling TAC-STIM
Operating Conditions (TAC-STIM Product)
-Range: -40°F to 140°F (-20°C to 60°C).
-Maximum Output: 30V (peak), 60mA (peak).
-Load Impedance: 450 to 550 Ohms.
-TAC-STIM produces an electrical signal consisting of five 5,000-Hz pulses, repeating at a rate of 25 Hz. The
waveform of the TAC-STIM pulse is approximately a sine wave.
Operating Conditions (Charging Case)
-Range: -40°F to 140°F (-20°C to 60°C).
-Only use the charging case indoors.
-Do not place any object except TAC-STIM on the charging surface.
-Maximum Output: 5.5V DC, 5W.
-Input: 100 to 240 VAC, 50 to 60 Hz, 0.4A max.
Storage/Transport Conditions
-TAC-STIM should be stored at room temperature away from moisture.
-Range: 32°F to 100°F (0°C to 38°C).
-Replace cap after each use.
-Store TAC-STIM in such a way (e.g., drawer or shelf) that the cap remains in place and is not accidentally
removed.
Service Life
-The service life of TAC-STIM is 3 years after the date the product has been activated.
-The expiration date of the conductive gel is 5 years after the date of manufacture.
Contact Information
Customer Experience Team:
E-mail: support@tac-stim.com
electroCore, Inc.
200 Forge Way, Suite 205
Rockaway, NJ 07866
United States
Telephone: 888-901-7846
Manufacturer:
E-mail: support@tac-stim.com
electroCore, Inc.
200 Forge Way, Suite 205
Rockaway, NJ 07866
United States
Telephone: 888-901-7846
Product Complaint Reports and/or related issues may be submitted directly to electroCore, Inc.:
Telephone: +1 (973) 355-6708
E-mail: complaints@electrocore.com
©2023 electroCore, Inc. All rights reserved. electroCore, the electroCore logo, TAC-STIM, and the TAC-STIM logo
are trademarks of electroCore, Inc. For patent information, please visit electroCore.com electroCore software or
firmware, or any updates or later versions thereof, included in or provided for use with any electroCore product is
provided subject to a revocable, non-exclusive license solely for use with such electroCore product to operate
such product for its intended use, and not for any other use, and may not be copied, altered, removed, modified,
reprogrammed, de-compiled or used for any other purpose. Any attempt to access, copy, remove, modify,
reprogram, de-compile, or otherwise use any software licensed hereunder in any manner inconsistent with this
license grant shall entitle electroCore to terminate the license.
Customer Service Limited Liability: electroCore guarantees against any out-of-box failures and warrants that the
Products shall meet the Product Standard. The warranty does not apply to any Product that: (i) has been
subjected to abuse, misuse, neglect, negligence, accident, improper testing, improper installation, improper
storage, improper handling, abnormal physical stress, abnormal environmental conditions, or use contrary to any
instructions issued by electroCore; or (ii) has been reconstructed, repaired, or altered by persons other than
electroCore or its authorized Representative. Customers shall not service, repair, modify, alter, replace, reverse
engineer, or otherwise change any Products.
64000-00195 Rev 1
Table of contents
Other ElectroCore Medical Equipment manuals
ElectroCore
ElectroCore gammaCore Sapphire SLC User manual

ElectroCore
ElectroCore gammaCore Sapphire User manual

ElectroCore
ElectroCore gammaCore Sapphire SLC User manual

ElectroCore
ElectroCore gammaCore Sapphire User manual

ElectroCore
ElectroCore gammaCore Sapphire SLC User manual
ElectroCore
ElectroCore gammaCore Sapphire CV User manual
ElectroCore
ElectroCore gammaCore-S User manual