ElectroMedic EVA User manual

EVA
INSTRUCTIONS
OPERATING
BEFORE
Portable, compact, easy-to-use devices
thateffectively respond to an even
broader range of perineal and pelvic
rehabilitation care, thanks to our close
andconstant collaboration with health
care professionals and their patients.
USER MANUAL
IN ENGLISH
NMESNMES
T E N ST E N S
STIMULATES and RELIEVES
a readaption at arms reach

2
1 INTRODUCTION BEFORE USING THE STIMULATOR
1.1 Introduction: A Close And Constant Collaboration With Health Care Professionals And Their Patients. 3
1.2 Medical Background. Use and Benets of Nerve Stimulation 4
1.3 Safety Measures. Indications. Contraindications. Precautions. Warnings. Adverse Effects 5-9
2INTRODUCING THE DEVICE
2.1 Equipment and Accessories 10
2.2 Technical Features, Electrical Specications 11
2.3 Preset Program Options 12
2.4 Keypad Functions 13
2.5 Screen Display 14
3INSTRUCTIONS
3.1 For the Patient 15-16
3.2 Special Instructions 17
3.3 Lock/Unlock a Program 18
3.4 Timer 18
3.5 Manual Controller 18
3.6 Stop/Pause an Ongoing Program 18
3.7 Li-ion battery - Li-ion battery charger/The patient, designated operator 19-20
4PROGRAMS 21
5CUSTOMIZATION
5.1 NMES 22
5.2 TENS 23
6PROGRAMMING CHART 24
7MAINTENANCE AND CLEANING 25
8TROUBLESHOOTING 26
9WARRANTY 27
10 FAQ 27
11 DOCUMENT HISTORY 28
12 LEGEND 29
page
5YEAR
WARRANTY
All Electro-Medic
devicescome with
a5(five) yearwarranty
that starts on the date
ofpurchase.
THE ELECTRO-MEDIC
WARRANTY
applies only to the
device, does not
cover any accessories
(wires,batteries,
charger), whichare
covered by a3(three)
month warranty.

2Portable, compact, easy-to-use devices that
effectively respond to an even broader range
ofphysical rehabilitation care, and particularly
forperineal andpelvicrehabilitation.
An electrotherapy culture meticulously designed
for women’s needs in relation to perineal and pelvic
rehabilitation and pain management for these areas.
A medically recognized therapy technique used both
by health care professionals in a clinical setting and
bypatients at home to ensure that the gains made
in the clinic are maintained.
BEFORE USING THE STIMULATOR
INTRODUCTION
Collaboration
1.1
3
page
1
Thank you for choosing
Electro-Medic, a proud Canadian
manufacturer of muscle stimulators
(NMES) and transcutaneous
electrical nerve stimulators (TENS),
offering you high-end devices
andaccessories on the cutting
edge of technology.
ELECTRO-MEDIC,
In partnership with
Service d’électro-Thérapie (SET)
Expert in Electrotherapy using
TENS and NMES, presents its
new muscle stimulator designed
forwomen’s pelvic and perineal
health, the EVA
Thanks to our close and constant collaboration
with health care professionals and their patients.

NMES
Transcontinuous neuromuscular stimulation is applied to normally
innervatedpelvic oor muscles.
The muscle bres are not directly stimulated; rather, the stimulation is
done through the nerve endings.
The electrodes or probe are applied to the muscles to be worked
insuch a way that the current stimulates the motor nerves and
induces a muscle contraction. For external use, one of the electrodes
will ideally be positioned on a motor point (the area that can most
easily be excited by the current). For internal use, the vaginal
or anal probe will be inserted and will be in contact with the pelvic
oor muscles.
To be effective, neuromuscular electrical stimulation requires
precise
adjustment of several parameters, such as: adjusted pulse shape,
sufcient and comfortable current intensity, and adequate frequency.
It is important, therefore, to use it under medical supervision,
asrecommended by a qualied health care professional.
TENS
Transcutaneous electrical nerve stimulation – TENS – involves
depolarizing peripheral nerve bres, using a current transmitted
bymeans of electrodes placed on the skin or internally by a probe.
TRANSCUTANEOUS ELECTRICAL NERVE STIMULATION
APPLIED AT A SENSORY LEVEL
The purpose of this stimulation is to enhance the effectiveness
ofthenatural pain control mechanisms. The current causes
atingling sensation which will then trigger a natural analgesic
reaction inthenervous system.
ACCESSIBLE TO ALL
The option of choosing a TENS program that is appropriate
forone's type of pain makes it a highly effective non-surgical,
non-drug therapy solution. TENS can be used to manage pain
bothduring activity andat rest, both in the clinic and at home.
In order to optimize the results of TENS nerve stimulation,
werecommend that you be under the care of a qualied health
careprofessional.
* For safe and proper use of the TENS, please follow the
recommendations of your qualied health care professional.
1. 2
NERVE STIMULATION
Its uses and benefits
Medical Context
N M E S
T E N S
4
page

TENS INDICATIONS
A safe, medically recognized therapy technique
for pain control with no side effects.
PAIN IN CHILDBIRTH AND POST-PARTUM PAIN
• Pain related to childbirth
• Pain related to Post-partum uterine contraction
• Postural pain (musculoskeletal) related to infant care
CHRONIC PELVIC PAIN SYNDROME
• Chronic vulvar pain syndrome (vulvodynia) and vestibulodynia
• Painful bladder syndrome (Interstitial cystitis)
• Endometriosis-related pain syndrome
• Dysmenorrhea related pain
• Deep dyspareunia
OVERACTIVE BLADDER
5
page
1. 2 1.3 SAFETY MEASURES
INDICATIONS, CONTRAINDICATIONS, PRECAUTIONS, WARNINGS, ADVERSE EFFECTS
NMES INDICATIONS
Neuromuscular electrical stimulation (NMES), particularly effective
forfacilitating strengthening and rehabilitation of muscles generally,
but also used widely in perineal and pelvic rehabilitation.
• Stress urinary incontinence
• Urge urinary incontinence
• Mixed urinary incontinence
• Fecal incontinence
• Pelvic oor weakness:
- Post-partum
- Chronic lower back pain
- Pelvic organ prolapse
• Hypertonic pelvic oor

6
page
CONTRAINDICATIONS (C-I)
• Malignancy/neoplasia: risk of spreading metastases. Risk of
increasing tumour growth. Cancer (or suspected cancer) is a local
contraindication toelectrotherapy currents; they may therefore be
used away from the affected site. In the case of metastases, use of
electric current isgenerally contraindicated. Patients who have already
had cancer arerecommended to wait until the remission period is
completed before restarting use of TENS or NMES on the affected
site. However,under certain conditions (e.g. palliative care), with
informed consent provided by the patient and by the interdisciplinary
team, useof TENS or NMES is possible.
• Cardiac pacemaker: absolute or local contraindication. Risk of
interference with the normal function of the cardiac pacemaker.
A cardiologist's permission is needed.
LOCAL CONTRAINDICATIONS
• Transcranial: risks of applying the electrodes transcranially are
unknown.
• Eyes: the risks of treating this part of the body are unknown.
• Anterior cervical region/carotid sinus: risk of stimulating the vagus
nerve, phrenic nerve, pharyngeal muscles or carotid sinus.
• Infection: the infection may spread.
• Skin weakened by radiation therapy: could stimulate the growth
ofremaining malignant cells.
• Damaged or delicate skin: resistance is decreased, which increases
the risk of burns.
• Do not apply stimulation to open wounds, erythema or rashes, or to
swollen, red, infected or inamed areas.
• Undiagnosed persistent pain.
LOCAL CONTRAINDICATIONS
• Do not apply stimulation to the patient's torso because the passage of
an electrical current through the chest can cause life-threatening heart
rhythm disturbances.
• Electronic implant: risk of interference with normal implant function.
• Heart disease: risk that the heart will have difculty keeping up with
the high metabolic demand. Patients with a suspected or diagnosed
cardiomyopathy should follow the recommendations of their doctor.
• Pregnancy: endogenous opiates released during muscle
contractions induced by electrical stimulation may stimulate
myometrial contractions. Electrical muscle stimulation of large
muscle groups should therefore be avoided during pregnancy
(NMES). Riskofaffecting the development and growth of the foetus.
Riskoftriggering premature uterine contractions. The effects
oftheuse in the perineal area during pregnancy are unknown (TENS
and NMES).
• Recent surgery, unstable fracture, osteoporosis: muscle contractions
could cause a muscle tear or even a displacement of the fracture.
• Epilepsy: local C-I on the head and neck. Precautions on the trunk
and extremities. Electrical stimulation could trigger a seizure.
• Sensory disorder: risk that the patient does not feel the current
adequately, which increases the risk of burns or skin irritation. (Lossof
sensation. Proceed with caution if stimulation is applied toareas of
the skin with a lower than normal level of sensation).
• DVT/blood clot/embolism: a blood clot could move into
thebloodstream.
• Tuberculosis: there is a risk of spreading infection.
• Bleeding (or risk of bleeding): risk of promoting bleeding.
DO NOT APPLY TO THESE REGIONS
OR USE UNDER MEDICAL OR
INTERDISCIPLINARY SUPERVISION

7
page
PRECAUTIONS
• Circulatory disorder: stimulation increases the metabolic demand and
this demand can exceed the oxygen supply, thus increasing the pain.
It can also lead to ischemia or necrosis in the tissue.
• Impaired cognition or communication: increased risk of injury
tothepatient. The patient's opinion, judgement and behaviour must
be known in order to use the device safely. (Do not apply stimulation
to patients who are unable to express themselves).
• Skin disease (e.g. eczema): resistance is decreased,
which increases the risk of burns.
• Active epiphyseal plate: risk of impairing bone growth.
• Chest: risk of affecting normal heart function.
• Lower abdomen: high-intensity stimulation carries a risk
of increasing gastrointestinal motility.
• Abundant fat tissue: risk of ineffective treatment; the current does
not reach the target tissue (muscle) because the fat tissueincreases
electrical impedance, which limits the penetration ofthe current
(NMES).
SPECIFIC CONTRAINDICATIONS
AND PRECAUTIONS FOR
INTERNAL USE
LOCAL CONTRAINDICATIONS
• Urethral pathology: stenosis, urethral stricture, irradiated
or scarred urethra
• Presence or suspicion of a vaginal, urinary or anal infection
or yeast infection
• Fistula, vulvar lichen sclerosus
PRECAUTIONS UNDER THE SUPERVISION
OFAQUALIFIED HEALTH CARE PROFESSIONAL:
• Systemic locomotor disorders
• Spinal conditions
• Post-partum: wait for 6 to 8 weeks before starting any
neuromuscular electrical stimulation
• Pelvic or abdominal surgery in the last 6 months
• Pelvic organ prolapse
• Acute urinary retention
* This device is not indicated
for stimulating denervated
pelvic oor muscles.

8
WARNINGS
•
Consult a qualied health care professional before using the device,
because the device may cause lethal heart rhythm disturbances
insome susceptible individuals.
•
Use this device only as recommended by a qualied health care
professional. (positioning of electrodes or probe, adjusting settings).
•
Never begin a rst stimulation session on a person who is standing
up. The rst ve (5) minutes of stimulation should be performed while
sitting or lying down. In rare cases, nervous individuals may suffer
a vasovagal reaction. This reaction is related to a fear ofmuscle
stimulation and shock at experiencing the unintentional muscle
contraction. A vasovagal reaction can cause the heart toslow
down and blood pressure to drop, which can lead to weakness
andsyncope. If this happens, stop stimulation. The patient should
liedown with his or her legs elevated until the feeling of weakness
goes away (5 to 10 minutes).
•
Long-term effects: we are unaware of any long-term effects of NMES.
•
Do not apply stimulation near any metal items. Remove all jewelry,
piercings, belt buckles or any other metal objects or devices in the
area of stimulation.
•
Abrupt changes in temperature can cause condensation to build
upinside the stimulator. To avoid this, allow the device to come back
to room temperature before using it.
•
During stimulation sessions, never disconnect a stimulation wire
while the stimulator is switched on. The stimulator should
be turned off rst.
•
During sessions, the stimulator should always be turned off before
moving or removing the electrodes.
•
Apply NMES only on normal, intact, clean and healthy skin.
•
Do not use electrodes with an active area of less than 2.54cm
indiameter; otherwise, skin burns may occur. Proceed with caution
ifthe electric current density is higher than 2 mA/cm².
•
Always use conductive gel with carbon electrodes or the probe
toavoid risk of skin damage.
•
The stimulator should be used only with electrodes or a probe that
are intended for stimulating nerves and muscles. Muscle pain may
occur after stimulation but generally disappears within a week.
•
Inspect the electrodes before each use. Change the electrodes when
they begin to wear out or lose adhesiveness. Poor contact between
the electrodes and the patient’s skin increases the risk of irritation
orburns on the skin. Apply the electrodes so that their entire surface
is in contact with the skin.
•
Do not share electrodes with other patients. Each user should have
apacket of electrodes and a probe in order to avoid any adverse skin
reactions or disease transmissions.
•
The manufacturer denies all liability in cases where electrodes are
positioned in ways other than as recommended.
page

ADVERSE EFFECTS
•
Patients may feel irritation and skin burns under the stimulation
electrodes applied to the skin.
•
Patients may experience headaches and other painful sensations
during or after the application of electrical stimulation near the eyes,
on the head or the face.
•
Patients should stop using the device and consult a doctor if they
experience an adverse reaction.
•
Some patients may experience extra sensitivity or skin irritation due
to the electrical stimulation or the electrical conductor (Gel). Irritation
may be alleviated by using a different conductor or by placing
theelectrodes differently.
•
Some patients may experience redness under the electrodes after
the session. This redness generally disappears within a few hours.
Ifskin redness persists after a few hours, the patient should consult
a doctor. Do not begin another stimulation session on the same area
if redness is still visible. Do not scratch red areas.
SAFETY MEASURES
•
Keep out of the reach of children.
•
Risk of electric shock.
•
Near other equipment. Do not use the device when it is placed
near to or above other equipment. If it is necessary to use it in such
asituation, make sure that ALL THE PERIPHERAL EQUIPMENT
ISOPERATING CORRECTLY under these conditions.
•
Do not use the device at the same time as monitoring equipment
(e.g. ECG equipment) that uses electrodes. The signals generated
bythe device could interfere with those of the monitoring equipment.
•
Accessories. Use this device only with manufacturer-recommended
electrodes, probes and accessories. Using other accessories may
harm the performance of the device, increase electromagnetic
emissions or reduce the electromagnetic immunity of the device.
•
Do not modify. No modications to the equipment are authorized.
•
Battery or stimulator heating up. Under extreme use conditions,
some parts of the casing may reach 43°C (109°F). Handle the battery
and hold the device carefully immediately after use. This temperature
may cause an unpleasant sensation but does not pose a particular
health risk.
•
Strangulation. Never wrap the wires around the patient's neck and
always keep out of the reach of children. Tangled wires may lead
tostrangulation.
•
Falls. Pay attention to wires on the ground to prevent falls.
•
Damaged device or accessories. Never use the device oraccessories
if damaged (casing, wires, etc.) or if the battery compartment is open
because there is a risk of electric shock. Carefully inspect the wires
and connectors before each use.
•
Foreign body. Do not allow any type of foreign body (dirt, water,
metal, etc.) to get into the device or the battery compartment.
•
LI-ION BATTERY. Do not carry the battery in a pocket, wallet orany
other place in which the terminals could cause a short-circuit.
Thismay generate intense heat and cause injury. Never open
the battery compartment cover during stimulation due to the risk
ofelectric shock. Remove the battery from the device if you do not
plan to use it for a long period, i.e., more than three (3) months.
Leaving the battery in the device for a long period may damage
thebattery and the device.
•
To avoid damage to the wires, it is best to leave them connected
tothe stimulator between two (2) sessions. Do not shake the wires
and connectors.
•
Equipment with internal power supply, type BF applied parts are
notsuitable for:
_
Use in the presence of a ammable anaesthetic mixture with air,
oxygen or nitrous oxide.
_ Continuous use
9
page
10
page
It is recommended
that only accessories
authorized by Electro-
Medic be used.
2
MDA534627
L’EXPÉRIENCEDU MOUVEMENT
STIMULE et SOULAGE
650, boul. Industriel Suite 100 Blainville Qc J7C 5Y7
GCAR-4060 4Rectangle 4 x 6 cm
CCAR-4040 4Carrée/Square 4 x 4 cm
Modèle / Model Q Forme / Shape Grandeur /Size
No. lot :
Exp :
Carbon
Electrodes
Électrodes
de carbone
GEL
GEL
Conducteur
Conductive
Pour électrodes | For electrodes
Hypoallergique
Formule hydrosoluble
pour l’électrothérapie
Hypoallergenic
Aqueous Electromedical
Coupling Agent
2015 - 001
GEL-90
3 . oz.(90ml)
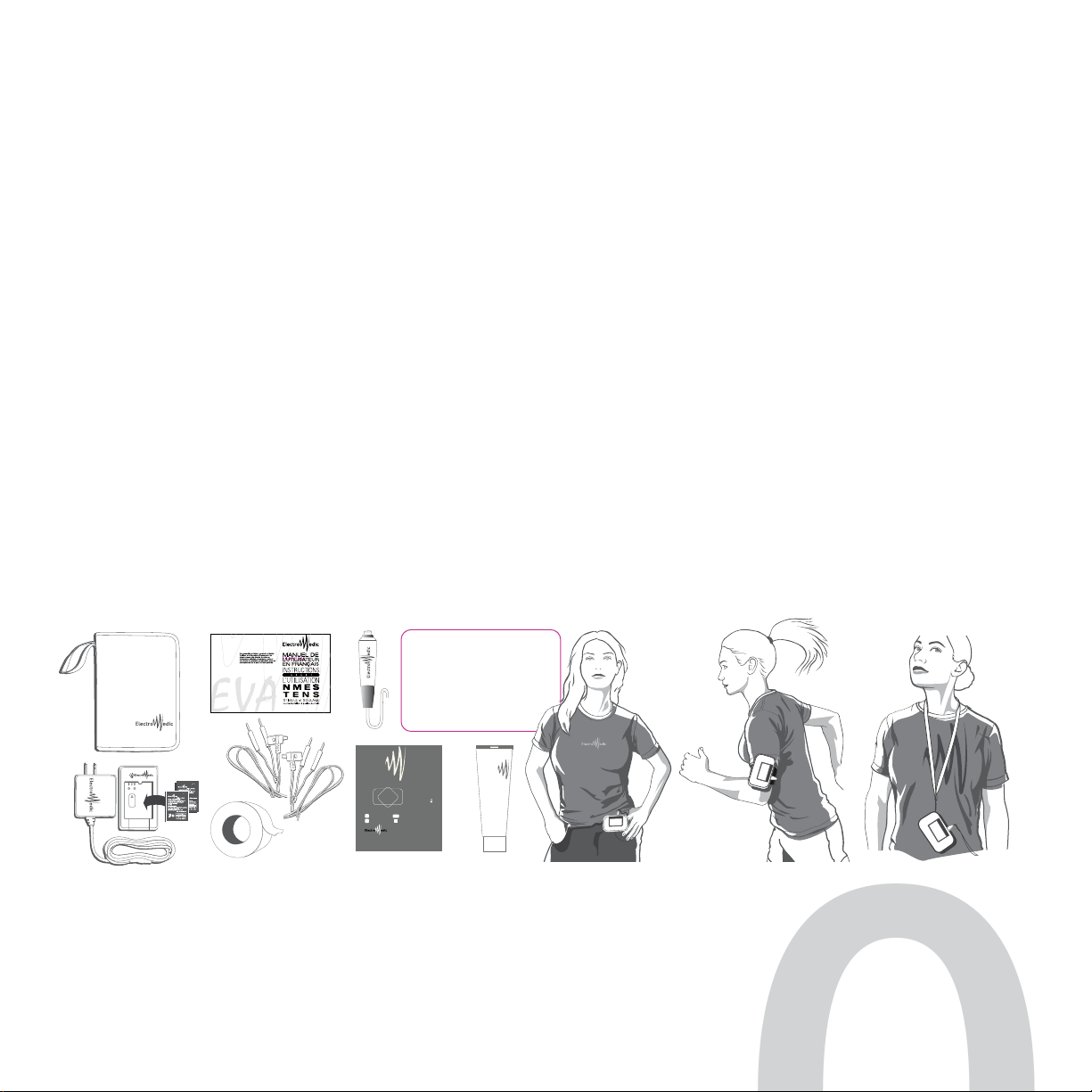
INTRODUCING THE DEVICE
2.1 EQUIPMENT AND ACCESSORIES |MODEL: EVA
THE EVA PORTABLE MUSCLE STIMULATOR COMES WITH MANY ACCESSORIES INCLUDING
•1case •1EVA NMES •1quick-start guide •1charger and 2Li-ion batteries •2wires and 1set of carbon electrodes
•1250 ml tube of gel •1roll of adhesive tape •1manual controller •1protective silicone sleeve which can be used with
•1belt clip and •1elastic band for attaching to an extremity for greater comfort and mobility •1neck strap for easily wearing
the NMES device
ATTENTION
Carefully read the instructions about using the electrodes as explained on their packaging.
USER
MANUAL
IN ENGLISH

10
TECHNICAL
FEATURES
A muscle stimulator designed for women’s pelvic and perineal
health, the Electro-Medic EVA stimulator has two channels,
designed for physical rehabilitation and particularly for pelvic
and perineal rehabilitation (NMES) and pain relief (TENS).
Thestimulator has 19 programs, including 7 that can be
customized. Electrical stimulation therapy requires a stimulation
current capable ofpenetrating the resistance of the skin and
theelectrode, i.e.approximately 1500 ohms.
The Electro-Medic muscle stimulator can penetrate this
resistance and maintain a current intensity of up to 100mA.
Achange inload from 100 to 1500 ohms results in less than
10% variation instimulation current from the set value.
The Electro-Medic muscle stimulator works with a rechargeable,
3.7V/600mAh Li-ion battery with a separate charger.
2.2
•Number of channels
•Constant current
•Stimulation current/channel
•Form of pulse
•Number of preset programs
•Number of customizable programs
•Form of stimulation
•Maximum pulse width
•Maximum frequency
•Timer
•Power supply
•Use
•Storage and transportation
•External dimensions
•Weight with battery
•Weight without battery
ELECTRICAL SPECIFICATIONS
2 non-independent in NMES mode
2 independent in TENS mode
1 manual controller
Up to a resistance of 1500 ohms
(an increase in load may reduce the maximum current)
From 0 to 100mA (maximum load: 40µ C)
Symmetrical biphasic pulse, 100% compensated
12
7
Continuous stimulation
Intermittent stimulation
Conventional (Continuous)
Burst
Pulse duration/modulated frequency
40-400 µs
1-150 Hz
From 1-60 min/Continuous (C)
1 rechargeable lithium-ion battery, 3.7 volts/600 mAh
+5°C to +40°C, 15% and 90 % R.H.: 700 hPa and 1060 hPa
-10°C to +60°C, 15% and 75% R.H.: 700 hPa and 1060 hPa
110 mm (L), 64 mm (W), 17 mm (D)
Approx. 114 g
Approx. 90 g
11
page
12
NMES
TENS
page
• Stress incontinence
• Urinary incontinence
• Mixed incontinence
• Stimulation of posterior tibial nerve
• Dysmenorrhea
• Muscle relaxant
• Conventional
• Burst
• Modulated pulse duration (MW)
• Massage
CHOICE
OF PRESET
PROGRAMS
2.3
12
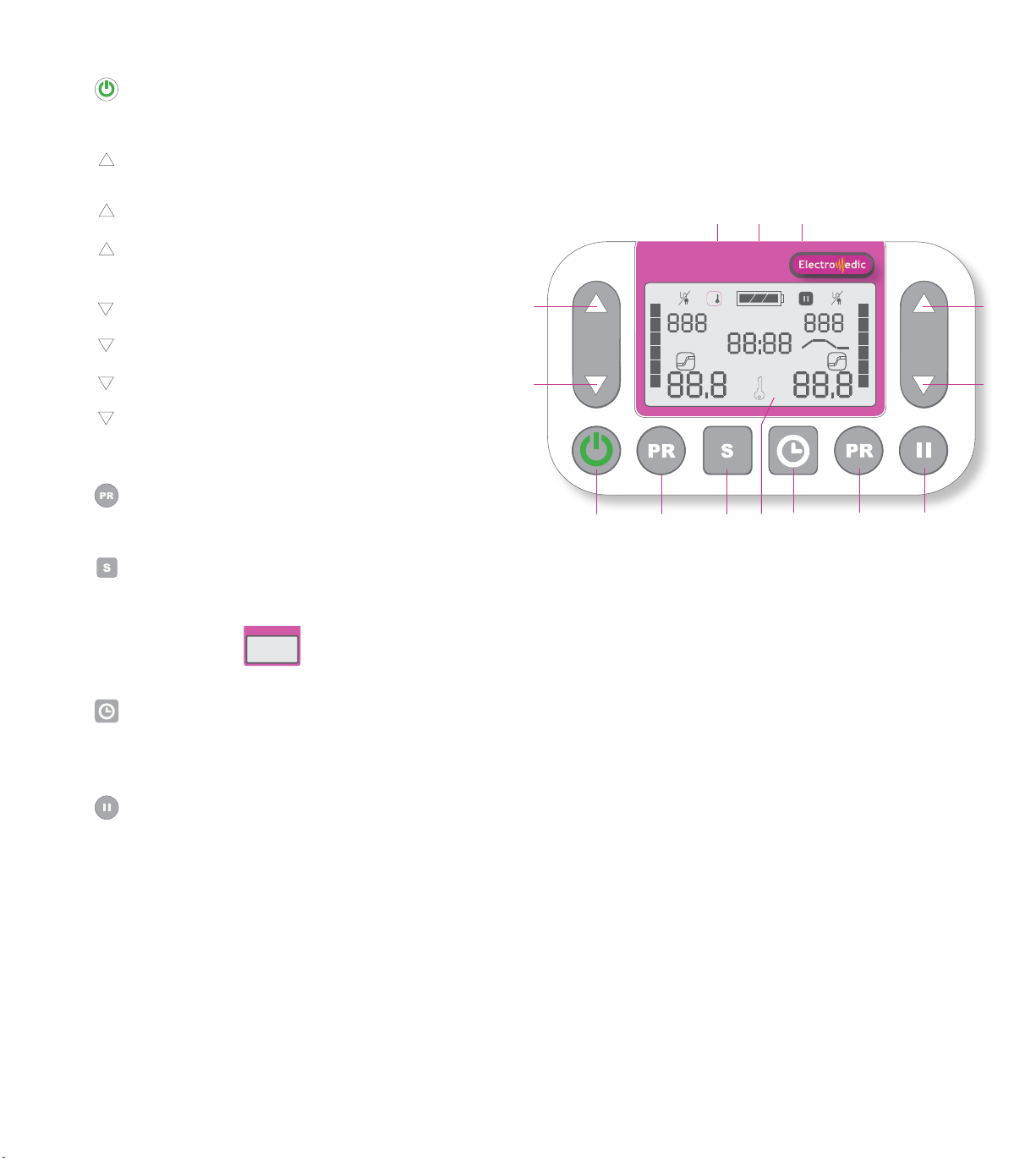
1 ON/OFF BUTTON
In addition to turning the device on and off,
allows stimulation to be stopped at any time.
2 3 FUNCTIONS
INCREASE
Allows the intensity of the left or right channel to be increased.
*Increases the intensity carefully, as prescribed.
CUSTOMIZABLE PROGRAMS
TIMER
Also allows the timer to be adjusted.
3 4 FUNCTIONS
DECREASE
Allows the intensity of the left or right channel to be reduced.
LOCK
Also deactivates the lock.
CUSTOMIZABLE PROGRAMS
Also allows you to switch from one program to another.
TIMER
Also allows the timer to be adjusted.
4 PROGRAMS
Choice of 12 preset programs and 7 custom programs
for safe andeffective customized treatment.
5 SELECTION
Hold the button down for 3 seconds to access
program customization mode.
Conrm, save the selection of the current program.
6 DIGITAL SCREEN
7 TIMER
Activates the timer, allows you to set the length of treatment.
Options: 1=60 min. timer or timer on continuous mode C
depending on your needs and the recommendations of your
health care provider.
8 PAUSE
Puts the device in standby mode, brings the intensity down to zero.
The intensity will then resume gradually when you press
the pause button. The timer stops when the device
is in pause mode.
9 CHANNEL #2 OUTPUT
10 CHANNEL #1 OUTPUT
11 MANUAL CONTROLLER CONNECTOR
13
page
22
33
1 4 5 6 47 8
9
10 11
Eva
CONTROL
BUTTONS
2.4
P
Hz2
ALT CB MXF
CMMRINT MW
µS
MANUAL CTRL
Timerhours
CH1 CH2
SI
ALT
T
SM
1
1
2
3
3
4
2
-
+

1OPEN CIRCUIT
•Electrode or probe disconnected
•Poor contact of electrode or probe
•Wire breakage
•Impedance too high
•Other likely issue
2PROGRAM
Displays the selected program.
•The left side displays the number of the channel 1 program
•The right side displays the number of the channel 2 program
3TIMER
Displays the remaining time.
4WORK/REST
-Work/rest indicator for intermittent stimulation programs.
-The upper part of the symbol blinks in work phase.
-The lower part blinks in rest phase.
5INTENSITY
Intensity of the channel in graduated scale.
6PULSE INTENSITY
Intensity of the channel represented in numbers.
7CHANNEL 1
8INDICATES THE STIMULATION MODE
9LOCK
Indicates whether the program is locked.
10 CHANNEL 2
11 INTERMITTENT STIMULATION PHASE
This symbol shows the 4 intermittent stimulation phases.
It will be displayed with customized programs that require
a rest period between muscle contractions.
THERE ARE 4 PHASES:
-1 - Ramp-up phase
-2 - Work phase
-3 - Ramp-down phase
-4 - Rest phase
1
2
34
P
Hz2
CB MXF
CMMRINT MW
µS
MANUAL CTRL
Timerhours
CH1 CH2
SI
ALT
T
SM
SCREEN
DISPLAY
2.5
17
1 1
16
15
14
13
12
11
2 2
3
4
4
55
6
89
6
18
710
12 µS OR Hz DISPLAY
In selection mode, indicates whether the numerical
value is in µs or Hz.
13 DEVICE IN PAUSE MODE
14 SI/ALT
-Symbol present during NMES muscle stimulation
only (SM mode)
-SI: Indicates that the 2 channels are working
simultaneously
-ALT: Indicates alternating between 2 channels
15 MANUAL CONTROLLER (MANUAL CTRL)
-Indicates that the stimulator is in manual mode
and controlled by the manual controller.
-When the manual controller is inserted into the
device, the symbol automatically appears.
16 BATTERY STATUS
Indicates the battery level in thirds
(1/3 - 2/3 - 3/3)
17 LOCKED PROGRAM
Program cannot be modied.
18 T or SM
Indicates which mode the device is in:
-TENS (T)
-NMES MUSCLE STIMULATOR (SM)
14
page

Do not place the device in a position
where it will be difcult to quickly
reach the main power source
to cut it off if needed.
DEVICE
SETUP
FOR THE PATIENT
INSTRUCTIONS
The Electro-Medic EVA
stimulator gives you the option
ofchoosing the treatment mode
thatbest suits your needs: treatment
byNMES orTENS. Inaddition, since
ithas 2 separate outputs, it allows
you to treat several parts of the
body atonce indifferent modes with
different intensities; some programs
and parameters can be modied under
thesupervision of a qualied health
care professional.
For optimal and completely safe
use, only use accessories provided
by Electro-Medic. In addition,
setting thedevice to the appropriate
intensity and only increasing the level
gradually will provide the comfort,
improvement and relief you seek.
Increasing levels too quickly is not
recommended.
3
N.B.
Only works for NMES
programs
Be careful when handling the wires toavoid
damaging them. The electrodes used
with this device must never be smaller
than 2.54 cm insize. Be aware that
thesmaller the electrodes are, themore
intense thestimulation will beatthesite
oftheelectrodes.
CONNECTING THE ELECTRODES
TO THE WIRES
B.1
Be careful when handling the wires to avoid
damaging them. Please make sure that the
probe is clean before use.
CONNECTING THE PROBE
TO THE WIRES
(probe not included)
B.2
(Please refer to the section:
REPLACING THE BATTERY
3.7 for more information)
INSERTING
THE LI-ION BATTERY
INTOTHE DEVICE
A
CONNECT THE WIRES TO THE UNIT–
NMES –
AT THE INPUT FOR EACH
CHANNEL
D
CONNECT THE MANUAL
CONTROL CONTROLLER
(ifrequired)
EPress the ON/OFF button
TURN ON THE DEVICE
F
-
+
15
page
Apply to non-
irritated skin
that has been
washed and
dried, for the
best adherence
and optimal
electrode
performance.
C.1 APPLY THE ELECTRODES
Insert the probe,
using the gel
(recommended
for maximum
performance).
Do not insert the
probe
too deeply.
For more detailed
information, please consult your
qualied health care professional.
C.2 INSERT THE PROBE
(probe not included)
3.1

16
page
To select a program, as recommended
by your qualied health care professional
for your diagnosed medical condition,
press the PR button PR
+or PR PR
-until
thedesired program is displayed in region 2
on the screen or press the arrow to start treatment.
For more detailed information onthe programs
available, refer to section 4: PROGRAMS.
STOP To stop stimulation, reduce the intensity with
thedown arrow until the intensity returns to 0.00
orpress the On/Off button.
SELECT A PROGRAM
(P1 to P19)
STOP STIMULATION
START STIMULATION
G
I
H
Press the INCREASE button for each channel until you reach
thepreferred level of stimulation. Press the button and hold it down
toincrease the intensity continuously.
N.B. Always increase amplitude carefully.
For the following cases, refer to section 3.2:
SPECIAL INSTRUCTIONS
• Intermittent stimulation + manual controller
LOCK The safety lock symbol indicates that the key lock is activated.
It will appear after 10 seconds once the intensity setting is selected.
So if you would like to increase the intensity, rst press the down arrow
todeactivate the safety lock. You can then adjust the intensity level
bypressing on the up or down arrows
Treatment length is preset but can be changed by using
the TIMER function

Intermittent stimulation (P1 - P2 - P5 - P6 - P7 - P12)
The programs indicated above include rest periods between muscle contractions (work phase) as illustrated below.
+ manual controller (P11)
OPERATION
The contraction increases progressively during the ramp-up phase and reaches its maximum during the work phase. During the ramp-down phase,
thestimulation reduces progressively until the start of the rest phase.
During intermittent stimulation programs, the work/rest symbol is displayed on the screen. The upper part of the symbol blinks
in work phase while the lower part blinks in rest phase.
For program 11, the manual controller is used to change intensity quickly during childbirth. There are two intensity settings for this program,
one for thecontraction phase and one for when the contraction has nished. Once the two intensity levels have been set, you only need
to press the button to toggle between them.
AMPLITUDE SETTING FOR CONTRACTIONS (work phase)
When the upper part of the work/rest symbol is blinking, increase the intensity gradually until you achieve painless muscle contractions.
3.2 SPECIAL
INSTRUCTIONS
1 1
2 2
3 3 4
4
• 1- ramp-up phase
• 2- work phase
• 3- ramp-down phase
• 4- rest phase
17
page

18
page
LOCK/UNLOCK
A PROGRAM
To lock or unlock a program, simultaneously press the down arrow of program 2 and the PR button of program 2 for 10 seconds.
You will see the lock symbol, illustrated above, in the section to the left.
•To change the lock status: use the down arrows to conrm the operation.
•If the lock symbol appears: program locked, cannot be changed.
•If the lock symbol does not appear: program unlocked; the user can change the program.
*Perform the same operation to unlock the program
TIMER
CHANGE THE LENGTH OF TREATMENT 0-60 MINUTES
•Press the Timer button and the timer will blink.
•The device will count down the elapsed time and will automatically stop once the time has run out
•For the continuous treatment option, without interruption, keep pressing until the signal Cappears. You will need to stop the device
yourself when you think the treatment has been long enough.Conrm the option selected by pressing the Sbutton to save,
or press the arrow to start the treatment
STOP/PAUSE
AN ONGOING PROGRAM
STOP
PAUSE
MANUAL CONTROLLER (MANUAL Ctrl)
FOR NMES ONLY
•To use the manual controller, connect the wire on top of your stimulator.
The message (MANUAL Ctrl) will appear on your screen.
•By pressing the button, you will be able to manually control your muscle contractions.
(Work Phase/Rest Phase)
For program 11 (pain relief in childbirth), the manual controller is used to change intensity quickly during childbirth. There are two intensity settings
forthis program, one for the contraction phase and one for when the contraction has nished. Once the two intensity levels have been set,
you only need to press the button to toggle between them.
For the other programs, the manual controller will be used to toggle between the contraction work phase and rest phase.
3.3
3.4
3.5
3.6
To stop the stimulation, reduce the intensity using the down arrow
until the intensity returns to 0.00 or press the On/Off button.
At any time during treatment, you can pause for 5 minutes.
•If the device is locked, unlock it by pressing the down arrow then pressing Pause
•The timer will stop while paused as desired
•To resume treatment, press Pause again

LI-ION BATTERY
You can always check your battery level by checking the following symbol:
BATTERY STATUS As displayed: 1/3 battery 2/3 battery 3/3 battery
BATTERY LIFE EXPECTANCY
The typical life of a lithium-ion battery is approximately: •Three (3) years
or
•300 charge cycles
a charge cycle refers to a complete discharge
followed by a complete recharge of the battery.
N.B. ONLY USE AN ELECTRO-MEDIC LI-ION BATTERY FOR THE STIMULATOR AND THE ELECTROMEDIC CHARGER TO RECHARGE THE BATTERY.
LI-ION BATTERY CHARGER
LED INDICATOR LIGHT FEATURES
•Adaptor (Model: JKY36-MDA534627)EN
•Input: 100 V-240 V~, 50/60 HZ, 150 mA
•Output: 4.2 V , 650 mA
•Red light: recharging
•Yellow light: recharging
•Green light: no battery in charger or battery fully charged
18
3.7
The stimulator only works with
an Electro-Medic 4.2V lithium-ion
battery.
You may continue treatment as long as the
stimulator is operating normally. When the effect
ofstimulation diminishes or the stimulator turns off,
it is time torecharge the battery. If you are not using
thestimulatorfor a period of time (approximately
three months), it is preferable to remove the
stimulator battery.
REPLACING
THE BATTERY
CHARGER
•Input: 4.2V input
•Output: 4.2V output
•Green light: fully charged
•Green light: device plugged in without battery
19
page
S/N: 2345628960557
WARNING
MADE IN CHINA
MADE IN CHINA
MAYEXPLODE IF DAMAGE D
ORDISPOSED OF IN FIRE
Li-ion battery
Li-ion

20
page
Do not position the device
in a way that would make
itdifficult to reach the main
power source and that
might prevent the device
being switched off rapidly
ifneeded.
The operator must
respond immediately
Colours of indicator lights
and their meaning
The operator must
respond quickly
Ready to use
Other meaning
green
yellow
Other
red
THE PATIENT IS THE DESIGNATED OPERATOR
The patient can operate the buttons and change the battery under normal conditions
andcan maintain the device and its accessories according to the user guide
ATTENTION!
•Use only Electro-Medic rechargeable Li-ion batteries
•NEVER reverse the (+) and (-) terminals when connecting them or let the batteries
come into contact with metal objects (necklaces, hairpins, etc.)
• NEVER charge Li-ion batteries for longer than 72 hours
•The battery charger must comply with IEC 60601-1 standards
SAFETY MEASURES
•Do not expose the equipment to re, direct sunlight or other heat sources,
which may cause a re or explosion, or generate toxic gases
•Do not store or transport the device with metal objects
•Do not disassemble or modify the device components
•Avoid all contact with water or any other liquid
INSTRUCTIONS FOR USING THE CHARGER
•Insert a Li-ion battery. Align the connection terminals (+) and (-) correctly
•Plug the charger into a standard wall outlet
•A red or yellow LED indicates charging
•When fully charged, the LED turns green.
Unplug the charger and remove the battery
CHARGING TIME
•A Li-ion battery takes about 3.5 hours to charge.
LI-ION BATTERY
•Limited voltage: 4.2 V
•Rechargeable Li-ion battery: 3.7 V/600 mAh
ADAPTOR
•100-240 v 50-60 Hz, 1.2A
ATTENTION
•This equipment must never be connected with an adaptor other
than the adaptor provided with the Electro-Medic equipment.
SAFETY MEASURES
•Do not cause a short-circuit
•Do not expose the device to high temperatures
•Use the charger only as specically recommended
The adaptor is a 2MOPP piece of equipment
under IEC 60601-1-1.
Approval of the equipment is valid if used
incombination with the adaptor provided
with this equipment.
Type BF applied part:
Electrodes
IP22
Continuous operation
Internally powered
Electro-Medic device
Applied part
Electrical equipment
protected against
harmful ingress
ofwater or particulate
matter.
Operating mode
Note: Not intended to be sterilized.
Not for use in an
oxygen-rich environment.
Protection from
electric shock
SAFETY CLASSIFICATION
OFELECTRO-MEDIC EQUIPMENT
Table of contents
Other ElectroMedic Fitness Equipment manuals
Popular Fitness Equipment manuals by other brands

G-FITNESS
G-FITNESS AIR ROWER user manual

CAPITAL SPORTS
CAPITAL SPORTS Dominate Edition 10028796 manual

Martin System
Martin System TT4FK user guide

CIRCLE FITNESS
CIRCLE FITNESS E7 owner's manual

G-FITNESS
G-FITNESS TZ-6017 user manual

Accelerated Care Plus
Accelerated Care Plus OMNISTIM FX2 CYCLE/WALK user manual












