Envirotainer CryoSure X1 User manual
X1, X2, X5, X11
Doc No. UM-CRYO-3030
Version 2
www.envirotainer.com
CryoSure®systems
User Manual
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

Record of revision
RECORD OF REVISION
VER. NO. REVISION
2REVISED:
•1.1 Overview,Table 1 Product specification:
• Relocated info of amount of vials, and added additional info in new chapter
1.1.1 Payload.
• Renamed table 1 from “Measurements and weight” to “Product specification”.
• Updated product specification data.
•1.4 Contact: New chapter.
•2.2 Symbols on the CryoSure® system.
• Removed UN3373 information. Only applicable for customer when adding bio-
logical substance category B.
• Added UN3481 information. If system is equipped by Envirotainer with a track-
er, the safety sticker will be added from the start.
•3.3 Monitor the autonomy duration left on your CryoSure® system replaces
chapter “Remaining dry ice time” which required an user to weigh the system.
• General editorial improvements:
• Clarified/changed wording.
• The word “vessel” was changed to either dewar or ®system.
• The word “data logger” was changed to tracker.
• Changed header from “Returning of empty product” to 3.2.4 Returning of
the system.
• Updated front page illustration.
• Updated illustration in 1.2 Technical details.
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

TABLE OF CONTENTS
1. INTRODUCTION.......................................................................................................................... 1
1.1 Overview .................................................................................................................................1
1.1.1 Payload......................................................................................................................2
1.2 Technical details.......................................................................................................................3
1.3 Intended use............................................................................................................................4
1.4 Contact....................................................................................................................................4
1.5 Operating conditions.................................................................................................................4
2. SAFETY......................................................................................................................................... 5
2.1 Shipping regulations .................................................................................................................5
2.2 Symbols on the CryoSure® system............................................................................................5
2.3 Cleanliness of product ..............................................................................................................5
3. USING THE CRYOSURE® SYSTEM....................................................................................... 6
3.1 Preparing a shipment................................................................................................................6
3.2 Operating instructions...............................................................................................................6
3.2.1 Product loading...........................................................................................................7
3.2.2 Shipping of loaded product ..........................................................................................9
3.2.3 Product unloading .....................................................................................................10
3.2.4 Returning of the system............................................................................................. 11
3.3 Monitor the autonomy duration left on your CryoSure® system ..................................................12
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

Intentionally left blank
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst
1. Introduction
1. INTRODUCTION
The CryoSure®system is designed for global shipping and storage of pharmaceutical products, clinical trial
materials, vaccines, cell & gene therapy, biospecimens and specialty medicines that are required to be kept
below -70°C (-94°F).
The CryoSure®system uses a unique patented technology combined with dry ice. It is a reusable shipping
system which offers both mechanical protection of the shipped product and maintains load space temperature
below -70°C (-94°F) (average between -74°C and -80°C / -101°F and -112°F).
• Maintains temperature control consistently below -70°C (-94°F)
• Suitable for any destination, domestic or international, and by ground or air, covering the entire shipment
duration including delays and customs without the need to re-charge
• Provides mechanical protection during transport by ground or air
The CryoSure®system comes in four different sizes, with product space volume from 1 L to 10.8 L. The
temperature control duration is intended to last throughout the shipment without recharging.
These systems will be pre-charged with dry ice at an Envirotainer facility and be delivered to a second location
where temperature-sensitive materials can be loaded into the dewar for transit or storage.
1.1 OVERVIEW
The CryoSure®product line consist of four different sizes.
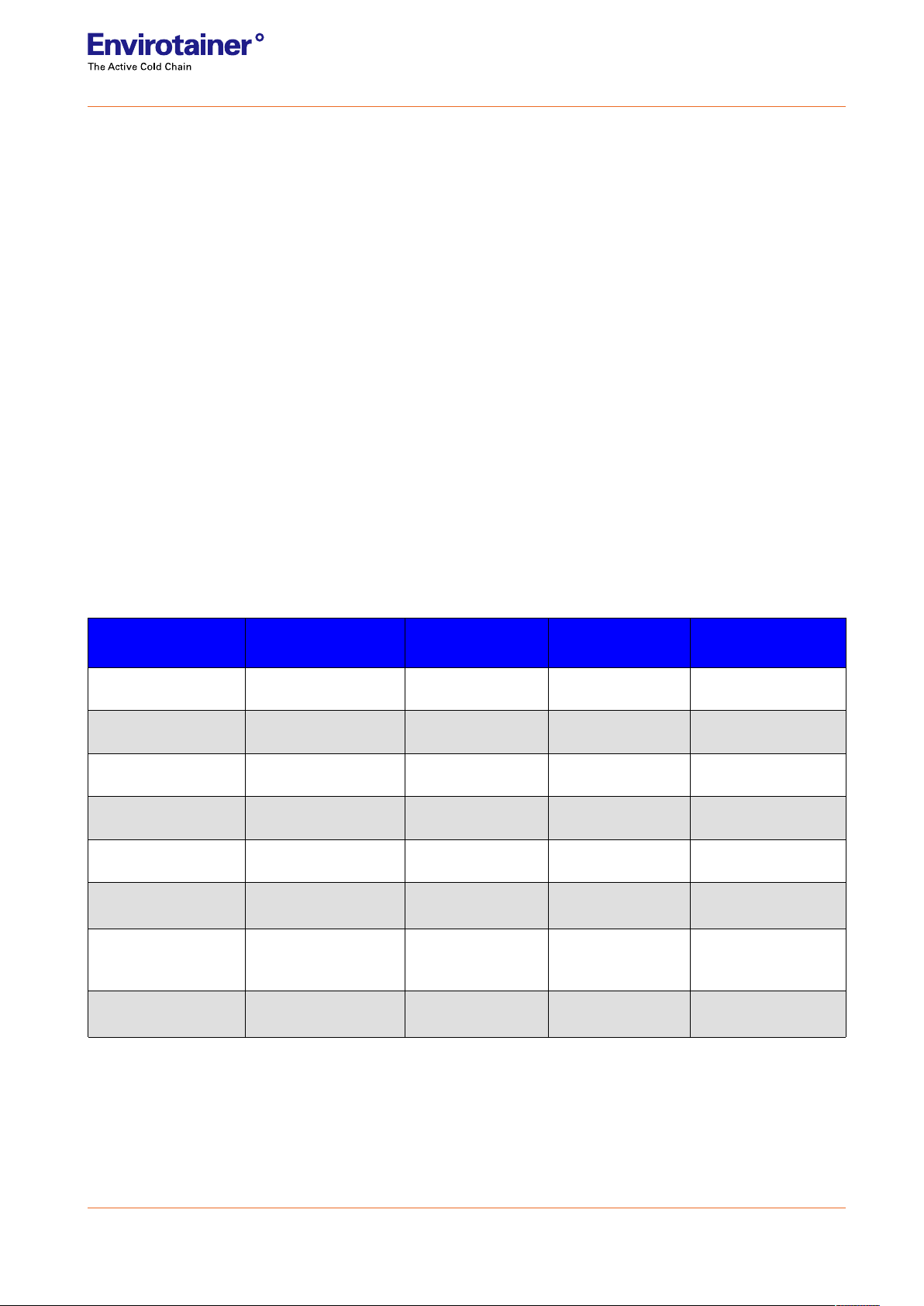
Table 1 Product specification
CryoSure°
system
X1 X2 X5 X11
Autonomous
duration* >8 days >21 days >13 days >15 days
Average
sublimation rate 0,0055 kg / hour 0,0063 kg / hour 0,010 kg / hour 0,012 kg / hour
Payload opening Ø 9 cm
(Ø 3.5ʺ)
Ø 9 cm
(Ø 3.5ʺ)
Ø 15.2 cm
(Ø 6ʺ)
Ø 21,3 cm
(Ø 8.4ʺ)
Dry-ice payload 1,2 kg
(2.5 lb.)
3,5 kg
(7.7 lb.)
3,2 kg
(7.2 lb.)
5,5 kg
(12 lb.)
Product payload 1 L
(33.7 oz.)
1.6 L
(55 oz.)
5 L
(170.3 oz.)
11.1 L
(376.3 oz.)
Product space
(DxH)
8,1 x 19,5 cm
(3.2ʺ x 7.7ʺ)
8,1 x 31,9 cm
(3.2ʺ x 12.6ʺ)
14,3 x 31,5 cm
(5.6ʺ x 12.4ʺ)
20,7 x 33,2 cm
(8.1ʺ x 13.1ʺ)
Outer dimensions
of box (WxLxH)
27,5 x 27,5 x 42,4 cm
(10.8ʺ x 10.8ʺ x 16.7ʺ)
30 x 30 x 59 cm
(11.8ʺ x 11.8ʺ x
23.2ʺ)
33,8 x 33,8 x 59 cm
(13.3ʺ x 13.3ʺ x
23.2ʺ)
45,7 x 45,7 x 63,5 cm
(18ʺ x 18ʺ x 25ʺ)
Total weight
(excl. product load)
4,8 kg
(10.6 lb.)
9,9 kg
(21.7 lb.)
11,3 kg
(24.8 lb.)
16,8 kg
(37 lb.)
* When exposed to ISTA 7D summer profile
1 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst
1.1.1 Payload
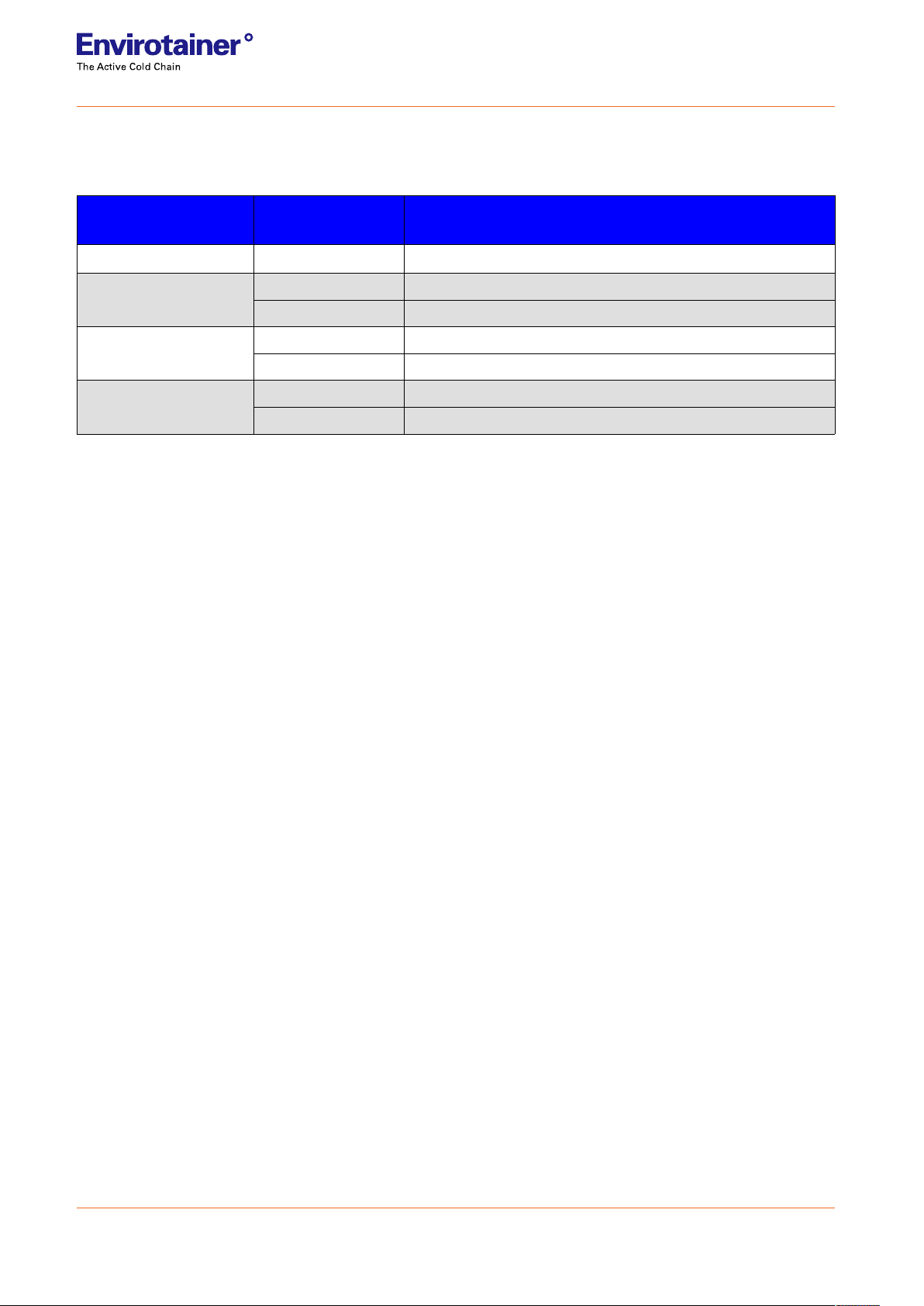
1.1.1 PAYLOAD
Table 2 Vials configuration
CryoSure®
version # of 2 mL vials 2 mL vials configuration
CryoSure®X1 20 Canister holding 2 bags; 10 vials/bag
CryoSure®X2 80 Canister holding 16 canes without sleeves; 5 vials/cane
70 Canister holding 14 canes with sleeves; 5 vials/cane
CryoSure®X5 300 Canister holding 60 canes without sleeves; 5 vials/cane
200 2 boxes oriented vertically; 100 vials/box
CryoSure®X11 600 120 canes without sleeves in bags; 5 vials/cane
500 Rack holding 5 boxes; 100 vials/box
2 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst
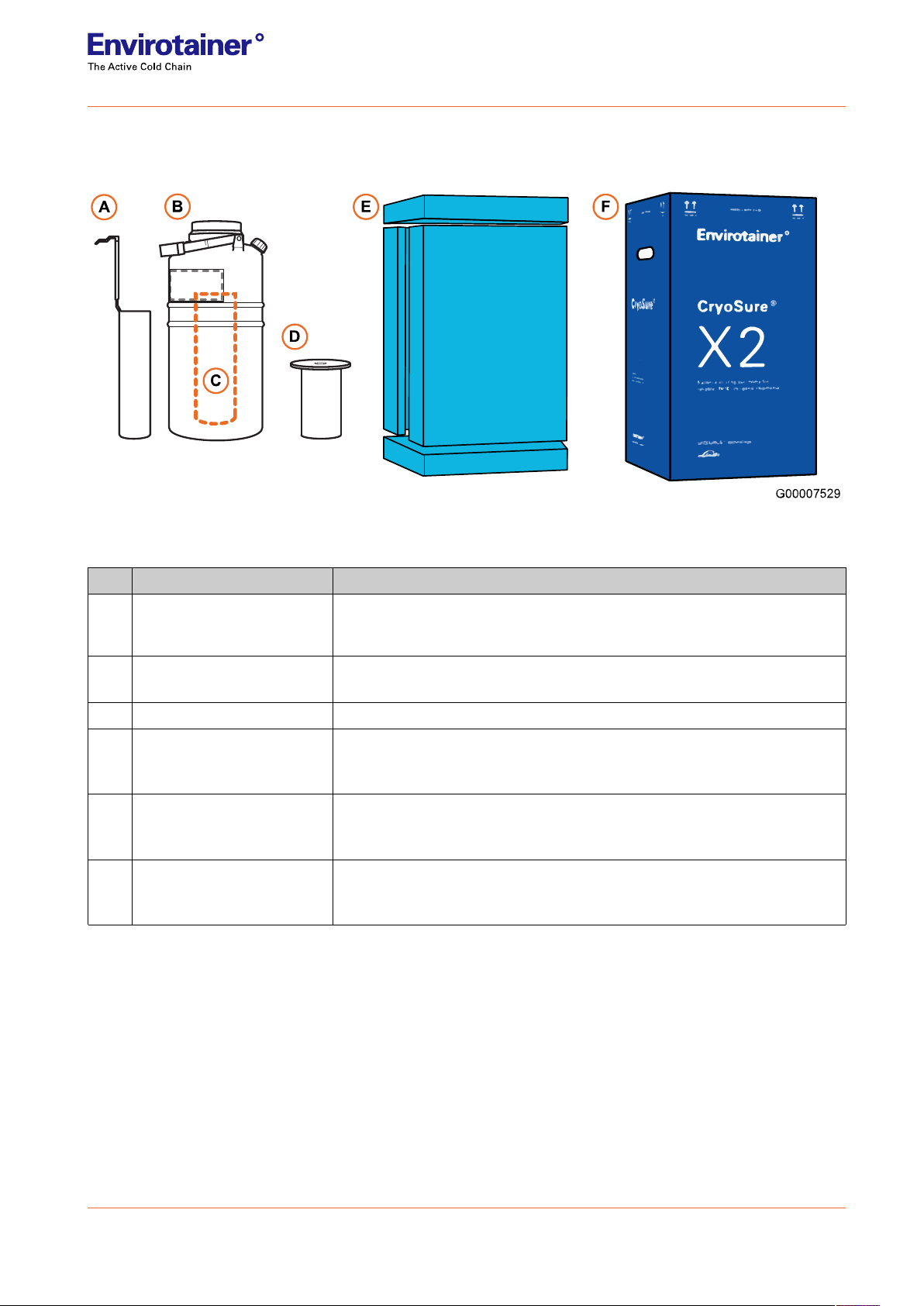
1.2 Technical details
1.2 TECHNICAL DETAILS
A complete CryoSure®system is comprised of the following components:
Fig. 1 The CryoSure®system.
Table 3 CryoSure®system features
Pos Name Description
AProduct holder
(standard for X2 or X5)
Cylindrical stainless-steel container with handle for loading materials into,
removing materials from, or holding materials within the storage zone of
the dewar
BDewar Insulated aluminum container that holds the dry ice refrigerant and
material to be preserved
CProduct space To place product holder in
DInsulating lid
Cap and insulating cork used to close the dewar opening while allowing
carbon dioxide vapor from sublimating dry ice inside the dewar to still
vent freely outside the dewar
EFoam inserts
Reusable packaging positioned between the dewar and cardboard box
that protects the dewar from handling damage:
(one (1) bottom, one (1) top, and two (2) side pieces)
FCardboard box
External packaging
Shipping, safety, and regulatory labels are applied to the exterior of the
cardboard box as appropriate
3 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

1.3 Intended use
1.3 INTENDED USE
Pre-charged CryoSure®systems are delivered with dry ice already loaded inside. In normal operation, users are
never required to handle dry ice.
The CryoSure®system is designed for global shipping and storage of pharmaceutical products, clinical trial
materials, vaccines, cell & gene therapy, biospecimens and specialty medicines that are required to be kept
below -70°C (-94°F).
The CryoSure®system offers both mechanical protection of the shipped product and maintains load space
temperature below -70°C (-94°F) throughout the shipping duration.
Refer to 2. Safety.
IMPORTANT!
Biological materials used with the CryoSure®systems are limited to Biological Substance Category B
materials specified under UN 3373 and exceptions defined in IATA 3.6.2.2.3.
No pathogenic Category A materials (UN 2814, UN 2900) or medical or clinical wastes (UN 3291) shall
be used with the CryoSure®systems.
1.4 CONTACT
For contact information to our operations centers, refer to www.envirotainer.com.
For questions regarding this manual or the CryoSure®systems, send an e-mail to support@envirotainer.com.
1.5 OPERATING CONDITIONS
Ambient temperatures, humidity, improper orientation of system and mechanical shock does not impact the
CryoSure®system temperature levels.
4 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

2. Safety
2. SAFETY
• Read the manual before handling and operating the CryoSure®system.
• Read the Safety Data Sheet for dry ice (UN1845).
• Pay attention to warning stickers and texts attached to the CryoSure®system.
2.1 SHIPPING REGULATIONS
The United States Department of Transportation (DOT) and related international government agencies regulate
shipments containing dry ice and biological materials. In addition, the International Air Transport Association
(IATA) maintains standards that guide airline polices regarding dry ice and biological material shipments. Users
of a pre-charged CryoSure®system should review and follow all appropriate government and carrier
requirements for labeling and packaging shipments containing dry ice or biological materials.
2.2 SYMBOLS ON THE CRYOSURE®SYSTEM
Fig. 2
IMPORTANT!
UN1845 (Dry ice) is solidified carbon dioxide. At atmospheric pressure, dry ice sublimates to carbon dioxide
vapor at approximately -78.5°C (-109.3°F). Dry ice is very cold, and contact with dry ice or areas cooled by
dry ice can cause frostbite. The CryoSure®pre-charged system is delivered with dry ice already loaded inside.
In normal operation, it should not be necessary for users of a pre-charged CryoSure®system to handle dry
ice. The interior spaces and surfaces of a pre-charged dewar are very cold, and users are advised to wear
appropriate gloves when interacting with the interior of the dewar or items recently removed from the interior.
The carbon dioxide evolved by sublimation of dry ice can create potentially hazardous carbon dioxide-
enriched and/or oxygen-deficient atmospheres. Users of dry ice or dry ice-containing systems are
recommended to evaluate the potential for atmospheric hazards in their working environment and take
appropriate steps to address such hazards (for example, by using air monitoring devices and adequate
ventilation).
UN3481 Lithium ion batteries contained in equipment or packed with equipment. If the CryoSure®system is
equipped with a tracker, a safety sticker will be added by Envirotainer° to the system. Any other tracker or
data logger added to the system by the customer, containing Lithium batteries, must be equally declared.
2.3 CLEANLINESS OF PRODUCT
The CryoSure®system is cleaned using VIRKON™ solution and Isopropyl alcohol according to Envirotainer
instruction SOP-ENV-0229 and SOP-ENV-0234.
5 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

3. Using the CryoSure®system
3. USING THE CRYOSURE®SYSTEM
3.1 PREPARING A SHIPMENT
It is recommended to have the product pre-conditioned to -70°C (-94°F) or below -78.5°C (-109.3°F) for the
CryoSure®system to operate within the specified temperature regulating duration. Using the CryoSure®to cool
the product will reduce the temperature regulating duration.
3.2 OPERATING INSTRUCTIONS
1. Product loading, 3.2.1 Product loading.
2. Shipping CryoSure®system, 3.2.2 Shipping of loaded product.
• Remove old labels and documents.
• Prepare new labels and documents for cardboard box.
3. Receiving CryoSure®system and removing products, 3.2.3 Product unloading.
4. Returning/shipping CryoSure®system to Envirotainer/new destination, 3.2.4 Returning of the system.
• Remove any biological product materials and associated packaging from the dewar.
• Remove old labels and documents.
• Prepare new labels and documents for cardboard box.
6 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst
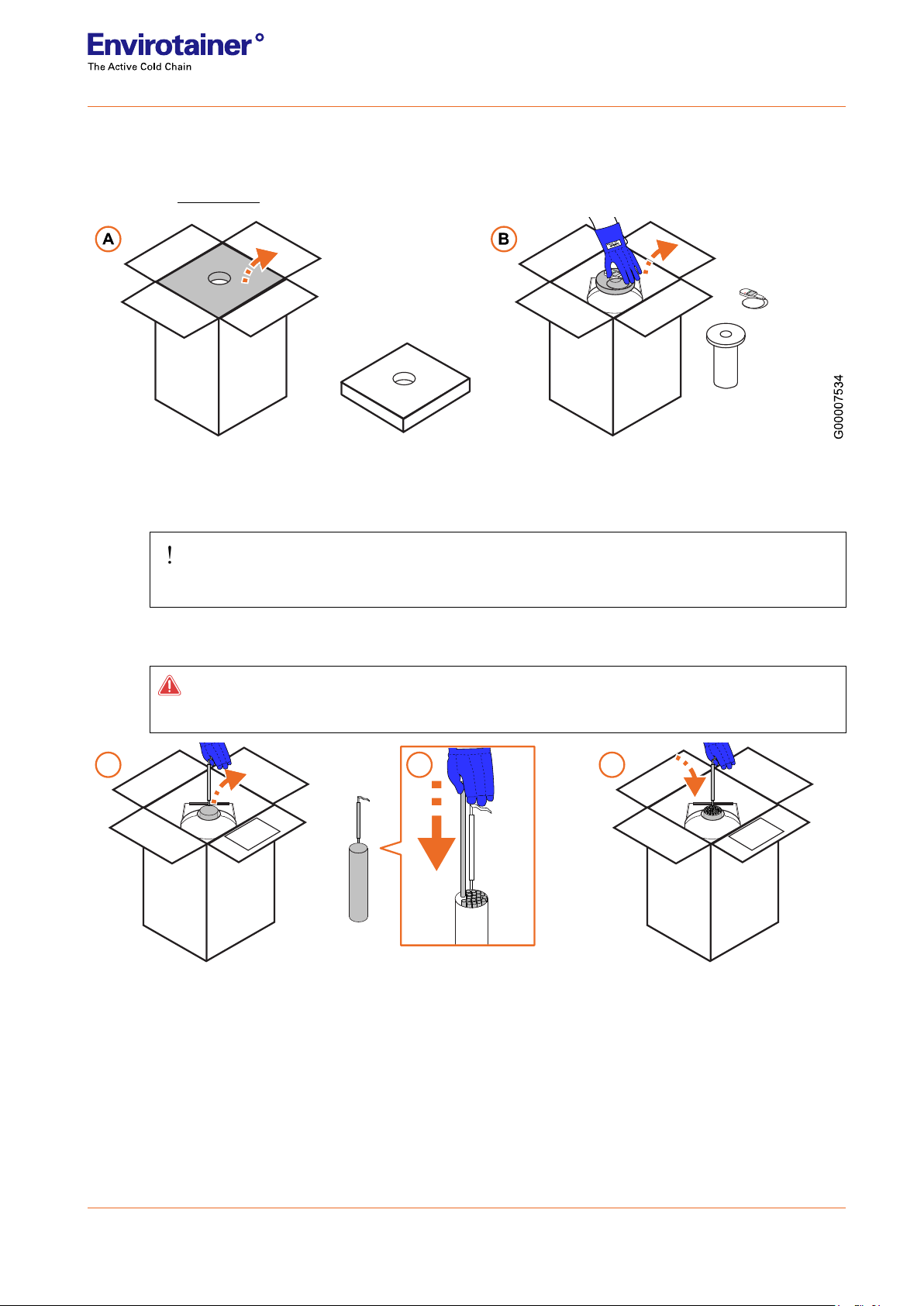
3.2.1 Product loading
3.2.1 PRODUCT LOADING
1. Inspect the outer packaging. If the cardboard box is too worn for use, contact Envirotainer for support.
Refer to 1.4 Contact
Fig. 3
2. Opening the box:
2a. Open the box and set aside the top foam.
IMPORTANT!
DO NOT DISCARD THE FOAM
2b. Wearing safety gloves, remove the lid. Place the lid to one side, together with the tracker and probe (if
any).
DANGER!
DO NOT DISCARD THE LID
Fig. 4
3. Loading the product:
3a. Lift the product holder by its handle and remove it from the dewar.
3b. Load the product into the product holder. Follow all applicable government and carrier requirements for
packaging the product.
3c. Lift the product holder into the dewar.
→
A C
G 00007532
B
7 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

3.2.2 Shipping of loaded product
3.2.2 SHIPPING OF LOADED PRODUCT
IMPORTANT!
After the dewar has been loaded with product and properly packaged with all required components, labels
need to be applied to the exterior of the cardboard box in accordance with government
(e.g., US DOT, EU ADR) regulations and carrier (e.g., IATA) requirements.
Please ensure that all needed documents according to the correspondent regulations are on hand (for
example shippers declaration, pro forma invoices and packing lists).
1. Remove or cover all labels on the cardboard box:
• the label of the parcel carrier
• the dangerous goods label (UN1845) where you are notified as consignee
Fig. 6
2. Apply the labels to the exterior of the box in accordance with government regulations and carrier
requirements.
3. Ensure that the following documents are in the folder:
• original shipper’s declaration (according to US DOT, EU ADR or IATA regulations)
• original pro-forma invoice
• original packing list
4. Close and seal the cardboard box with a clear tape.
5. Take necessary steps in order to inform selected carrier that the parcel is ready for pick-up.
9 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

3.2.3 Product unloading
3.2.3 PRODUCT UNLOADING
Fig. 7
1. Unpacking:
1a. Open the box and set aside the top foam.
IMPORTANT!
DO NOT DISCARD THE FOAM
1b. Wearing safety gloves, remove lid. Place the lid to one side.
CAUTION!
DO NOT DISCARD THE LID
Fig. 8
2. Unloading product:
2a. Lift the product holder by its handle and remove it from the dewar.
2b. Remove the product from the product holder. Store or use the product as defined by procedures.
3. Prepare the next shipment.
• If loading new product into the dewar, follow step 3b and onwards of the 3.2.1 Product loading
procedure to prepare the shipment.
• If sending the CryoSure®system to its next destination, follow step 3c and onwards of the 3.2.1 Product
loading procedure to prepare the shipment.
10 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst
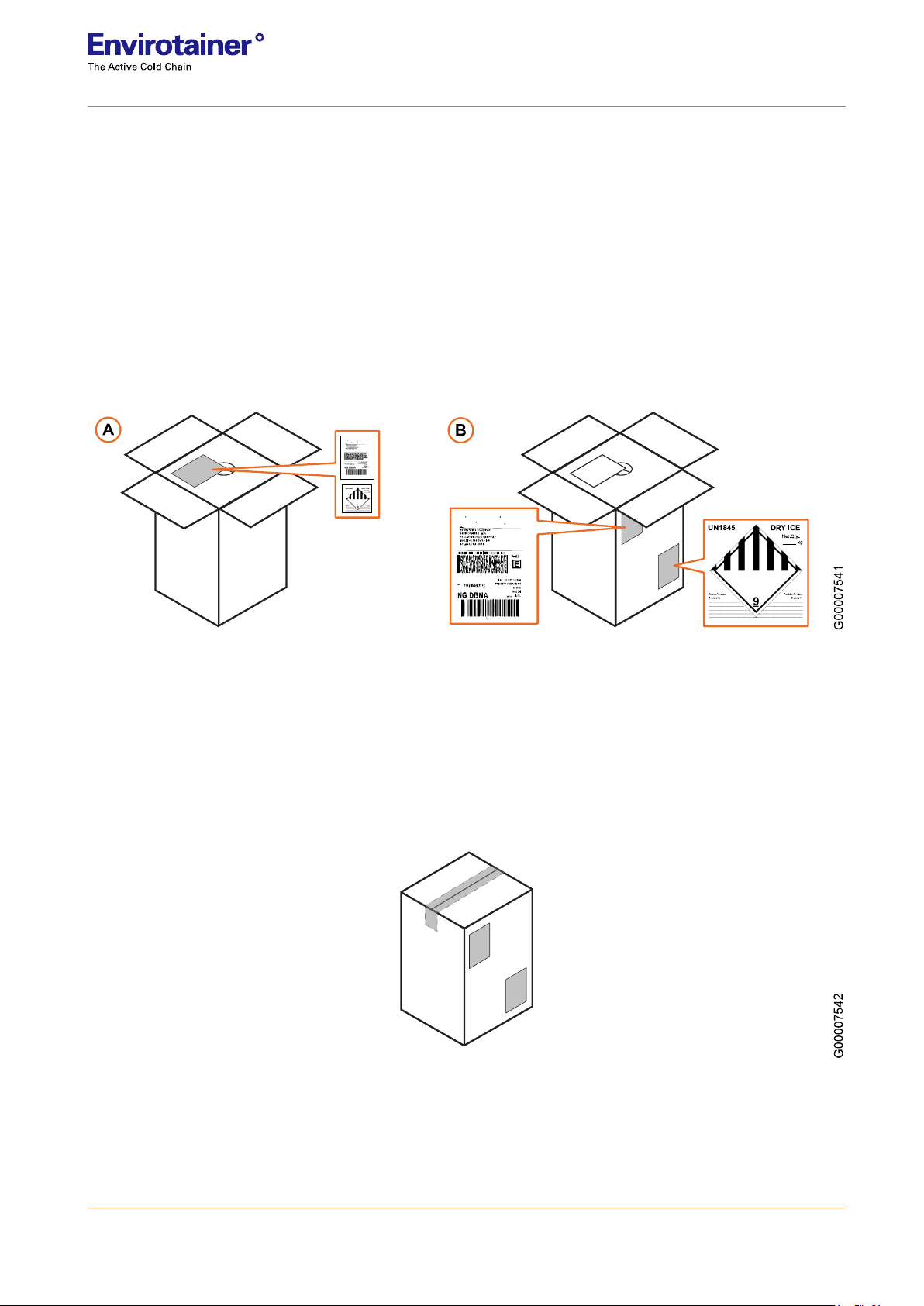
3.2.4 Returning of the system
3.2.4 RETURNING OF THE SYSTEM
If you have booked a pick-up service from Envirotainer, a prepaid shipping return label is included in a folder on
the inside of the cardboard box.
If you have not pre-booked the pick-up service, contact Envirotainer support to place an order for the service.
Otherwise, follow steps 1and 2below to prepare the CryoSure®system for return.
1. Remove any biological product materials and associated packaging from the dewar.
2. Remove or cover all labels on the cardboard box:
• the label of the parcel carrier
• the dangerous goods label (UN1845) where you are notified as consignee
• the dangerous goods label (UN 3373)
Fig. 9
3. Preparing the documents and labels.
3a. Remove the folder “Return” from the cardboard box. In the folder you find the pre-printed labels you
need for returning the CryoSure®product:
• the pre-paid label of the parcel carrier, where you are notified as Sender
• the dangerous goods label (UN1845), where you are notified as Shipper
3b. Peel and apply both labels and put them on the cardboard box.
Fig. 10
4. Close the cardboard box, and seal with a clear tape.
5. Inform Envirotainer that the CryoSure®system is ready for pick-up.
Upon your advice, Envirotainer arranges with the parcel carrier a pick-up.
11 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

3.3 Monitor the autonomy duration left on your CryoSure®system
3.3 MONITOR THE AUTONOMY DURATION LEFT ON YOUR CRYOSURE®
SYSTEM
Every time a CryoSure®system is released, a unique QR code sticker is printed and placed on the outside of
the cardboard box. By scanning the QR code with your smartphone, you will get access to the DuraWatch®
service which provide information about this unique shipment, including how many days and hours of cooling
capacity is remaining.
In addition to cooling capacity remaining, DuraWatch®also provides:
• Contact details to schedule pickup of the CryoSure®system
• Shipping route information
• Product details (dewar dimension and other measurements)
• User Manual
The QR code can be scanned by anyone handling the CryoSure®system and not only the customer placing the
CryoSure®orders. However, information in DuraWatch®is non-sensitive. For any detailed information around a
CryoSure®order you still need to login to the Envirotainer Portal.
12 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

Intentionally left blank
13 (14)
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst

Envirotainer AB
www.envirotainer.com
Doc No:
UM-CRYO-3030
Revision:
2
State:
Released
Release Date:
2021-12-01
Approved By:
hanst
This manual suits for next models
3
Table of contents
Other Envirotainer Industrial Equipment manuals

Envirotainer
Envirotainer RKN e1 User manual

Envirotainer
Envirotainer RKN e1 User manual

Envirotainer
Envirotainer RAP e2 User manual

Envirotainer
Envirotainer RKN t2 User manual

Envirotainer
Envirotainer Releye RLP 160020R User manual

Envirotainer
Envirotainer RKN e1 User manual

Envirotainer
Envirotainer RKN t2 User manual
Popular Industrial Equipment manuals by other brands

SSP
SSP EDI Series operating manual

STOKVIS ENERGY SYSTEMS
STOKVIS ENERGY SYSTEMS ECONOPLATE E3 Series Installation, operation & maintenance documentation

Rapid
Rapid AB-Z Operation manual

Baileigh
Baileigh BB-7216M Operator's manual
ABB
ABB HT579538 Operation manual

Roemheld
Roemheld 8910-01-20-H operating instructions