eversense CGM Sensor User manual

User Guide
A guide for using the Eversense
Continuous Glucose Monitoring System
Mobile AppSmart TransmitterSensor
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 1LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 1 2/26/20 12:58 PM2/26/20 12:58 PM

LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 2LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 2 2/26/20 12:58 PM2/26/20 12:58 PM

Eversense Trademark
Eversense, Eversense Continuous Glucose Monitoring, Eversense CGM, Eversense Sensor, Eversense Smart Transmitter,
Eversense App and the Eversense logo are trademarks of Senseonics, Incorporated. Other brands and their products
are trademarks or registered trademarks of their respective holders.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 1LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 1 2/26/20 12:58 PM2/26/20 12:58 PM

Contents
Glossary 5
1. Introduction 7
Help and Support 7
Eversense CGM System Components 8
System Requirements 12
End User License Agreement
and Privacy Policy 12
Jailbroken Devices 12
Broken Screen or Button 12
Indications for Use 12
MRI Safety Information 13
Contraindications 14
What is Included in this Package 14
How to Use this User Guide 14
2. Benets and Risks 15
Risks and Side Eects 16
Warnings 17
Precautions 19
3. Getting Started 21
Charge your Smart Transmitter 22
Step 1. Download and Install the App 24
Step 2. Set up the App – Account
Creation, Pairing and Settings 25
4. Inserting and Linking the
Sensor 32
5. Using the
Smart Transmitter 35
Daily Use 36
Secure the Smart Transmitter over
Inserted Sensor 37
Turn the Smart Transmitter ON and OFF
40
Smart Transmitter Care and Maintenance
41
Battery Indicator 41
LED Status Indicators 42
6. Calibrating the System 43
Calibration Phases 45
How To Calibrate 47
7. Using the App 51
Check Your Mobile Device Settings 52
Get To Know the “My Glucose” Screen 53
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 2LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 2 2/26/20 12:58 PM2/26/20 12:58 PM

Trend Arrows 56
Understanding Treatment Decisions
with CGM 57
Discuss with Your Health Care
Provider 59
Making Treatment Decisions with
Eversense 61
Eversense Trend Arrows
and Treatment Decisions 63
What Would You Do 65
Trend Graph 69
Menu Options 70
Profile Picture 71
8. Customizing your Settings 72
Setting Glucose Alert Levels 74
Setting Glucose Target Levels 76
Setting Predictive Alerts 78
Setting Rate of Change Alerts 80
Setting System Information 82
Setting Sounds 83
Transmitter Disconnect Setting 84
Setting Temporary Profile 85
Logging out 88
9. Alert Descriptions 89
Alerts and Notifications 89
Alert History 91
Alert Descriptions and Actions 93
10. Event Log 109
Glucose 111
Meals 112
Insulin 113
Health 114
Exercise 115
11. Reports 116
Weekly Modal Summary 117
Glucose Pie Chart 118
Glucose Statistics 118
12. Share My Data 119
Eversense Data Management Software
(DMS) Program 119
Sync 120
My Circle 121
13. Product and General
Information on the App 122
14. Viewing Eversense Data
on the Apple Watch 124
Alerts and Notifications Displayed
on the Apple Watch 127
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 3LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 3 2/26/20 12:58 PM2/26/20 12:58 PM

15. My Circle 141
Remote Monitoring with Eversense CGM
System and Eversense NOW App 141
16. About the Sensor 147
Insertion Steps 148
Removal Steps 149
17. Travel 150
18. Troubleshooting 151
Smart Transmitter 151
Smart Transmitter Battery
and Charging 153
Connection with Smart Transmitter 154
Calibration 157
Alerts and Notifications 159
Glucose Readings 161
Making Treatment Decisions 162
Trend Arrows 163
App 163
Sensor 165
Events 167
Sync 167
Shortcuts 168
19. Device Performance 169
Clinical Study Performance 169
Eversense Accuracy to YSI in
PRECISION Study 171
Sensor Life 188
Safety 190
20. Technical Specications 191
Sensor 191
Smart Transmitter 192
Power Supply and Charger 193
USB Cable* for Charging and
Downloading 193
Electrical and Safety Standards 194
Symbols on the Eversense CGM
Mobile App 197
Symbols on Packaging and Devices 199
Eversense Smart Transmitter
Limited Warranty 201
Legal Notices 204
Apple Legal Notice 204
Google Legal Notice 204
About Bluetooth® 204
Bluetooth® Trademark 204
FCC Information 205
Index 206
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 4LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 4 2/26/20 12:58 PM2/26/20 12:58 PM

5
Eversense CGM User Guide
Glossary
Alert An alert warns you that a situation needs your
attention and that you should respond/take appropriate
action.
Blood Glucose Meter A commercially available device
used to measure glucose using a blood sample from a
fingerstick.
Bluetooth® A brand name for a wireless networking
technology that uses short wave radio frequencies (RF)
to connect mobile devices and other wireless electronic
devices.
Calibration Blood glucose reading from a fingerstick
sample entered in the Eversense App to check the
accuracy of the system. With the Eversense System,
there are two phases: Initialization Phase during which
4 fingerstick tests are required, and the Daily Calibration
Phase, during which 1 fingerstick test is required twice
daily.
CGM Continuous Glucose Monitoring. Continuously
monitoring your glucose levels from interstitial fluid
every few minutes.
Contraindication A condition or circumstance in which
a person should not use the device.
CT Computed Tomography
Do Not Disturb Mode (DND in the Eversense App)
When enabled, the mobile app will stop displaying
non-critical alerts, and the smart transmitter will stop
providing vibratory notifications for non-critical alerts.
Critical alerts will still be provided. Many mobile devices
have a separate Do Not Disturb Mode. Consult the
manufacturer’s instructions for more information.
Electromagnetic Interference A strong field of energy
generated by electrical or magnetic devices.
EULA End User License Agreement
Eversense App Software program that is installed on a
mobile device and is used to display CGM glucose data
sent from the smart transmitter.
Eversense DMS A web-based application compatible
with the Eversense App where your glucose data is
stored and can be viewed.
Eversense NOW A remote monitoring mobile
application that allows you to share your glucose data
with other people.
FAQ Frequently Asked Questions
Health Care Provider A physician, physician assistant,
and/or nurse practitioner who has successfully completed
the Eversense CGM Insertion and Removal Training
Program and has read and understood the Eversense
CGM Sensor Insertion and Removal Instructions.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 5LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 5 2/26/20 12:58 PM2/26/20 12:58 PM

6
Eversense CGM User Guide
“HI” Reading Indicates a sensor glucose reading is
> 400 mg/dL.
Hyperglycemia An episode of high blood glucose.
Hypoglycemia An episode of low blood glucose.
Interstitial Fluid (ISF) The fluid between cells in
the body. The Eversense CGM measures glucose from
an interstitial fluid sample, versus glucose in a blood
sample obtained from a fingerstick.
Jailbroken Device A device (iPhone or iPod) that has
been modified to remove the controls and limits set by
the original manufacturer.
LED Light Emitting Diode
Linked Sensor A sensor that is connected to a smart
transmitter.
“LO” Reading Indicates sensor glucose reading is
< 40 mg/dL.
Mobile Device A handheld device built on a mobile
operating system that runs the Eversense App and
communicates with the smart transmitter.
mg/dL Milligrams per deciliter, a unit of measure that
shows the concentration of a substance in a specific
amount of fluid. In some countries, including the United
States, glucose test results are reported as mg/dL,
indicating how much glucose is in the blood when using
a blood glucose meter, or how much glucose is in the
interstitial fluid when using some CGM systems, like the
Eversense CGM System.
mmol/L Millimoles per liter, a unit of measure that
shows the concentration of a substance in a specific
amount of fluid. In many countries, glucose test results
are reported as mmol/L, indicating how much glucose is
in the blood when using a blood glucose meter, or how
much glucose is in the interstitial fluid when using some
CGM systems, like the Eversense CGM System.
MRI Magnetic Resonance Imaging
Rate of change/trend arrows Indicators of direction
and speed of change of your glucose levels.
Remote Monitoring An optional feature that allows
you to invite others to view your CGM data using
Eversense NOW, a separate mobile app they download
to a compatible mobile device.
Sensor A device inserted subcutaneously for
continually measuring interstitial fluid glucose levels.
Snooze Setting Used to set how often an alert repeats.
Subcutaneous Located beneath the skin.
Smart Transmitter A reusable device worn externally
over the inserted sensor that powers the sensor and
sends glucose information to the mobile device for
display in the Eversense App.
Warm-Up Phase The period the sensor requires to
adjust after the sensor has been inserted and before
calibrations.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 6LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 6 2/26/20 12:58 PM2/26/20 12:58 PM

1
7
Eversense CGM User Guide
1. Introduction
This section reviews how to use this guide and describes your new Eversense CGM System,
including its components and intended purpose.
Help and Support
Please review this User Guide with your health care provider. For additional Eversense product questions and
troubleshooting issues, contact Customer Support toll free in the US at 844-SENSE4U (844-736-7348). To download a
copy of this User Guide in Spanish, please visit www.eversensediabetes.com.
Congratulations on having Eversense CGM technology to assist you in managing your diabetes. Your Eversense CGM
System is intended to continually measure glucose levels for up to 90 days after your sensor is inserted. Glucose
information collected by the system is automatically sent to your mobile device. You must contact your health care
provider (physician, physician assistant, and/or nurse practitioner) to schedule the insertion and removal of your
sensor.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 7LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 7 2/26/20 12:58 PM2/26/20 12:58 PM

1
8
Eversense CGM User Guide
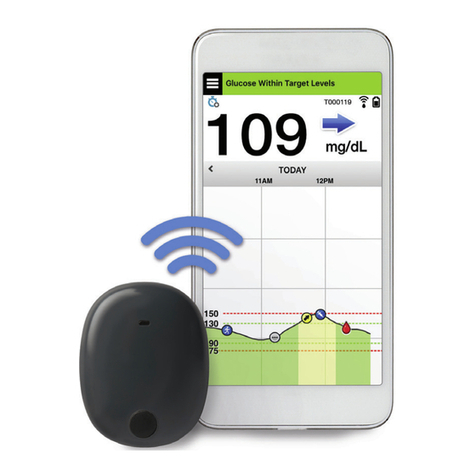
Eversense CGM System Components
The System includes 1) a small sensor inserted subcutaneously by a health care provider, 2) a removable smart
transmitter worn over the sensor, and 3) a mobile app to display the glucose readings.
Eversense Sensor
The sensor is inserted under the skin (upper arm) and measures glucose in
interstitial fluid for up to 90 days. These glucose levels are then calculated by
the smart transmitter and sent to the app.
Eversense Smart Transmitter
The removable smart transmitter is worn externally over the sensor and
powers the sensor. It wirelessly sends glucose data (via Bluetooth) to the
mobile device app. The smart transmitter also provides on-body vibe alerts
based on the glucose settings you choose. It has a rechargeable battery and
is reusable for up to one year. Smart Transmitter
Sensor
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 8LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 8 2/26/20 12:58 PM2/26/20 12:58 PM

1
9
Eversense CGM User Guide
Eversense App
The Eversense App is a software application that runs on a mobile device (e.g., smartphone or tablet) and displays
glucose data in a variety of ways. It also provides alerts based on the glucose settings you choose.
IMPORTANT: In order to use the Eversense CGM System, you must have an understanding of downloading
and using mobile apps on your handheld device. Data from the Eversense Smart Transmitter is sent wirelessly via
Bluetooth. Carefully read the instructions in this User Guide for downloading and installing the Eversense CGM Mobile
App, and for pairing your mobile device with the smart transmitter. If there is anything you do not understand in this
User Guide, please consult your health care provider. For product questions, contact Senseonics Customer Support.
Disposable adhesive patches for daily use are also included as part of the system, and will be provided to you by your
health care provider after your sensor has been inserted. The patch has an adhesive side that attaches to the back of
the smart transmitter, and a silicone adhesive side that attaches to the skin.
The Eversense App screens layout will vary based on
your mobile device’s model and/or operating system.
Throughout this User Guide, we have included some
examples of these dierences.
Make sure your mobile device is using the latest
operating system.
iOS Android
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 9LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 9 2/26/20 12:58 PM2/26/20 12:58 PM
1
10
Eversense CGM User Guide
Eversense System Overview
A separate blood glucose monitoring system (not provided by Senseonics) is required for calibrating the CGM System,
and to make treatment decisions. When used properly, these components work together to help ensure you get
continuous glucose monitoring for up to 90 days.
To ensure you receive continuous glucose readings and other information, follow these daily use tips:
Wear your smart transmitter all the time except when charging.
The smart transmitter is water-resistant to a depth of 1 meter (3.2 feet) for 30 minutes. Exposing the smart
transmitter to conditions beyond this will result in damage and void your warranty.
Make sure your smart transmitter has enough battery power at all times.
Perform two blood glucose meter calibration tests each day when prompted.
Pay attention to alerts and notifications you receive from your smart transmitter and mobile device.
Replace the adhesive patch on your smart transmitter daily.
You can remove the smart transmitter from the upper arm at any time, except during calibration. Remember that
no data are collected when the smart transmitter is not communicating with the sensor. When you place the smart
transmitter back on the sensor site, it will take 10 minutes for sensor communication to re-start and for glucose
readings to appear in the app.
When the smart transmitter and mobile device are not within range of each other, any data gathered by the smart
transmitter is stored and sent to the app when the mobile device and smart transmitter are back within range.
It is safe for you to wear your sensor and smart transmitter when you go through metal detectors at airports. While
flying, the smart transmitter performs similar to any other Bluetooth device. Be sure to follow the specific safety
guidelines mandated by the airline.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 10LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 10 2/26/20 12:58 PM2/26/20 12:58 PM

1
11
Eversense CGM User Guide
Some of the features of the Eversense CGM System:
•Wireless communication with the sensor, smart transmitter and app.
•Long-term sensor wear in the upper arm for up to 90 days.
•Alerts when pre-set Low or High Glucose Alert levels (hypoglycemia or hyperglycemia) are reached.
•Predictive Alerts let you know before reaching pre-set Low or High Glucose Alert levels.
•Use of mobile device (e.g., smartphone) to display glucose readings.
•On-body vibe alerts with the smart transmitter even when mobile device is not nearby.
•Provides readings within 40 - 400 mg/dL range every 5 minutes.
•Trend arrows that show whether your glucose values are rising or falling and how fast.
•Graphs and statistics that show your glucose results in easy-to-understand formats.
•Removable and rechargeable smart transmitter.
•Event entry capabilities (like meals, exercise and insulin).
•Stores glucose data in the app and on the smart transmitter.
•Provides remote monitoring capability to others using the Eversense NOW Mobile App.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 11LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 11 2/26/20 12:58 PM2/26/20 12:58 PM

1
12
Eversense CGM User Guide
System Requirements
•The Eversense CGM System.
•A compatible smartphone for Android (version 4.4 or higher) or Apple iPhone® or iPod® or iPad® (iOS version 8.0 or
higher) that has Bluetooth Smart (or Bluetooth Low Energy). The Eversense App also works with the Apple Watch®.
•For a list of compatible devices, please go to www.eversensediabetes.com.
•The Eversense App downloaded to your mobile device from the Apple App Store or on Google Play™.
End User License Agreement and Privacy Policy
Use of the Eversense App is subject to the terms and conditions of the most current Eversense App End User License
Agreement and Eversense App Privacy Policy. These documents are updated from time to time and are posted at
www.eversensediabetes.com.
Jailbroken Devices
DO NOT use the Eversense Apps on jailbroken iPhones or iPods. Jailbroken devices do not provide an acceptable level
of security for the user and are not approved for use by Senseonics.
Broken Screen or Button
If the screen of your mobile device is broken, or the buttons do not work, then you may not be able to use your
Eversense System and you may miss low or high glucose events.
Indications for Use
The Eversense CGM System is indicated for continually measuring glucose levels in adults (18 years or older) with
diabetes for up to 90 days. The system is indicated for use to replace fingerstick blood glucose measurements for
diabetes treatment decisions.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 12LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 12 2/26/20 12:58 PM2/26/20 12:58 PM

1
13
Eversense CGM User Guide
The system is intended to:
•Provide real-time glucose readings.
•Provide glucose trend information.
•Provide alerts for the detection and prediction of episodes of low blood glucose (hypoglycemia) and high blood
glucose (hyperglycemia).
The system is a prescription device. Historical data from the system can be interpreted to aid in providing therapy
adjustments. These adjustments should be based on patterns and trends seen over time.
The system is intended for single patient use.
MRI Safety Information
Non-clinical testing has demonstrated the Eversense Sensor is MR Conditional. A patient with this device can be safely
scanned in an MR system meeting the following conditions:
•Static magnetic field of 1.5T or 3.0T.
•Maximum spatial field gradient of 2000 gauss/cm (20 T/m).
•Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 W/kg (First Level
Controlled Operating Mode).
Under the scan conditions defined above, non-clinical testing results indicate the Eversense Sensor is expected to
produce a maximum temperature rise of less than 5.4 °C after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately 2.83 inches (72 mm) from the
Eversense Sensor when imaged with a gradient echo pulse sequence and a 3T MR system.
The Eversense Smart Transmitter is MR Unsafe and MUST BE REMOVED before undergoing an MRI procedure. Before
you undergo an MRI procedure, tell the MRI sta that you have an Eversense Sensor and Smart Transmitter.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 13LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 13 2/26/20 12:58 PM2/26/20 12:58 PM

14
Eversense CGM User Guide
What is Included in this Package
This Eversense Smart Transmitter Pack contains the following:
Also included in this package is this User Guide, Quick Reference Guide and a wallet card (not shown).
How to Use this User Guide
This guide describes how to use your CGM System. Read the entire guide before using the system.
•Any warnings or precautions are highlighted in a box.
•User tips are preceded by the symbol.
Eversense Smart Transmitter Power Supply
(USB cable and AC power adapter)
Charging Cradle
1Contraindications
The smart transmitter is incompatible with magnetic resonance imaging (MRI) procedures. The smart transmitter is
MR Unsafe and MUST BE REMOVED before undergoing an MRI (magnetic resonance imaging) procedure.
For information on the sensor, please see MRI Safety Information.
The system is contraindicated in people for whom dexamethasone or dexamethasone acetate may be contraindicated.
Mannitol or sorbitol, when administered intravenously, or as a component of an irrigation solution or peritoneal dialysis
solution, may increase blood mannitol or sorbitol concentrations and cause falsely elevated readings of your sensor
glucose results. Sorbitol is used in some artificial sweeteners, and concentration levels from typical dietary intake do
not impact sensor glucose results.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 14LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 14 2/26/20 12:58 PM2/26/20 12:58 PM

2
15
Eversense CGM User Guide
2. Benets and Risks
This section describes the benets, expectations and risks associated with using the Eversense
CGM System.
Continuous glucose monitoring aids in the management of diabetes and glucose control, which can improve your
quality of life. Best results are achieved when you are fully informed about the risks and benefits, insertion procedure,
follow-up requirements, and self-care responsibilities. You should not have the sensor inserted if you cannot properly
operate the CGM System.
The CGM System measures glucose in interstitial fluid (ISF) between the body’s cells. Physiologic dierences between
ISF and blood from a fingerstick may result in dierences in glucose measurements. These dierences are especially
evident during times of rapid change in blood glucose (e.g., after eating, dosing insulin, or exercising), and for some
people, during the first several days after insertion due to inflammation that may result from the insertion procedure.
Glucose levels in ISF lag behind glucose levels in blood by several minutes.
IMPORTANT: If your symptoms do not match the glucose alerts and readings from the Eversense CGM
System, a fingerstick blood glucose check with a home blood glucose meter should be performed prior to making
treatment decisions.
Failure to use the Eversense CGM System in accordance with the instructions for use may result in you missing a
hypoglycemic or hyperglycemic glucose event, which may result in injury.
The sensor has a silicone ring that contains a small amount of an anti-inflammatory drug (dexamethasone acetate).
It has not been determined whether the risks associated with injectable dexamethasone acetate apply to the
dexamethasone acetate elution ring inside the sensor. The elution ring releases a small amount of dexamethasone
acetate when the sensor comes in contact with body fluids and serves to minimize the body’s inflammatory response
to the inserted sensor. Dexamethasone acetate in the ring may also cause other adverse events not previously seen
with the injectable form. For a listing of potentially adverse eects related to dexamethasone acetate, contact your
health care provider.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 15LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 15 2/26/20 12:58 PM2/26/20 12:58 PM

2
16
Eversense CGM User Guide
Unauthorized modifications of the equipment, improperly accessing information within it or “jailbreaking” your
system, and taking any other unauthorized actions may cause the CGM system to malfunction and may put you at
risk. Unauthorized modification of the equipment is not permitted and voids your warranty.
Caution: Federal (US) law restricts this device to sale by or on the order of a physician.
Risks and Side Eects
The glucose alerts and notifications will not audibly notify the user when the sound on the mobile device is turned
o. If the system cannot display a glucose value, it also cannot provide glucose alerts. If you are unable to feel the
vibration of the smart transmitter you may not notice the alerts. You may need medical attention in the event that
you have high or low glucose and are unaware of it.
IMPORTANT: If you do not test your glucose with a blood glucose meter when your symptoms are not
consistent with the sensor glucose readings, you may miss a high or low glucose event.
Treatment decisions should be made based on a review of the following: a sensor glucose value, trend arrow, recent
glucose trend graph, and system alerts/notifications. You should not make a treatment decision unless you have
considered all this information.
Be sure you talk with your health care provider about insulin action, so you understand how its impact on your
glucose may factor into your treatment decisions.
The sensor is inserted by making a small incision and placing it under the skin. This process may cause infection, pain
or skin irritation. Additionally, the adhesive may cause a reaction or skin irritation.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 16LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 16 2/26/20 12:58 PM2/26/20 12:58 PM

2
17
Eversense CGM User Guide
Warnings
•The Eversense CGM System has not been tested using insertion sites other than the upper arm.
•If at any time your symptoms are not consistent with the sensor glucose readings, you should test your
glucose with a blood glucose meter.
•Before making a treatment decision, you should take into account the sensor glucose value, the trend graph,
the trend arrow and any alerts from the Eversense CGM System. If no trend arrow is displayed, the system
does not have enough data to display direction and rate of change. You should not make a treatment decision
based solely on the sensor glucose value.
•If your smart transmitter is damaged or cracked, DO NOT use, as this could create an electrical safety hazard
or malfunction, and could result in electrical shock.
•Close contact with direct EMI may interfere with the smart transmitter’s ability to send data to your mobile
device. Move away from the source of EMI and check that your mobile device is connected to your smart
transmitter.
•Antibiotics of the tetracycline class may falsely lower sensor glucose readings. You should not rely on sensor
glucose readings while taking tetracyclines.
•The bandage should remain covering the incision for 48 hours as this is a standard of care to allow formation
of a water-tight seal to help protect against infection. Until it has healed, always cover the insertion site with
a sterile bandage before placing the smart transmitter adhesive over the sensor. Failure to do so could result
in infection at the insertion site.
•Please review this User Guide with your health care provider. For additional Eversense product questions and
troubleshooting issues, contact Customer Support toll free in the US at 844-SENSE4U (844-736-7348).
•Always calibrate the system using only a fingerstick blood sample. DO NOT use an alternative site (such as
forearm or palm) blood glucose reading to calibrate the system.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 17LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 17 2/26/20 12:58 PM2/26/20 12:58 PM

2
18
Eversense CGM User Guide
Warnings (continued)
•DO NOT insert your infusion set or inject insulin within 4 in (10.16 cm) of the sensor site. If the insulin delivery
site is within 4 in (10.16 cm) of the sensor site, it may interfere with sensor glucose readings and can cause
inaccurate glucose readings.
•Always follow your health care provider’s instructions for care after the sensor insertion or removal. Contact
your health care provider if any of the following events occur:
– You have pain, redness, or swelling at the incision site(s) later than 5 days after the sensor insertion or
removal.
•If your sensor glucose is very low (below 40 mg/dL) or very high (above 400 mg/dL), you should perform a
fingerstick blood glucose test prior to making a treatment decision.
•The Eversense CGM System requires calibration in order to provide accurate readings. You should not use CGM
readings to make treatment decisions unless you have followed the instructions for daily calibration.
•The Eversense CGM System will not provide readings during the 24 hour Warm-Up Phase and until a second
calibration is successful during the Initialization Phase. During this time, you should monitor your glucose
using a home blood glucose monitor.
•Certain conditions and alerts will prevent glucose data from being displayed. During these times, you should
use a home blood glucose monitor to make treatment decisions. You should carefully read the Alerts and
Notifications section of their Eversense CGM System User Guide to understand these conditions.
•The glucose alerts and notifications will not audibly notify you when the sound on your mobile device is
turned o. If the system cannot display a glucose value, it also cannot provide glucose alerts. If you are
unable to feel the vibration of the smart transmitter you may not notice the alerts.
•When the smart transmitter is not worn over the sensor, such as during charging, the Eversense CGM System
will not provide alerts and notifications on the mobile device or through vibration alerts from the smart
transmitter.
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 18LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 18 2/26/20 12:58 PM2/26/20 12:58 PM
Other manuals for CGM Sensor
2
Table of contents
Other eversense Blood Glucose Meter manuals