FEAS 3850 User manual



DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
i
DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC
USER MANUAL

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
ii
IMPORTANT!
DEFIBRILLATOR MONITOR Mod. 3850B-BIPHASIC is intended for use by physician/medical trained in the operation of the
equipment and expertise in advance cardiac life support, defibrillation procedures, cardioversion, monitoring vital signs and pacing. It
can also be operated bya paramedic or nurse, underthe direct supervision and order of a physician/medical, and who has, at a
minimum, the following skills and training:
•CPR training.
•ACLS training equivalent to that recommended by the American Heart Association.
•Training inthe use of the DEFIBRILLATOR MONITOR Mod. 3850B-BIPHASIC.
This device is rated IPX2 per IEC529.
RESPONSIBILITY FOR INFORMATION
It is the responsibility of our customers to ensure that the appropriate person(s) within their organization have access to this
information; including general safety information providedin section“NOTES ANDWARNINGS”.
VERSION HISTORY
This User Manual (ref. 16184/0903B - MANUAL DE USO DESFIBRILADOR 3850 BBIFASICO INGLES) describestheDefibrillator
Monitor Mod 3850B-Biphasic with software version 3.27.
You can verifythe software version of thedevice during power up.The software version is displayed onscreen.
Edition Date: 2019/04/01.
MANUFACTURER
Av. Colón 5756/60 –C.P.X5003DFP –
CÓRDOBA – REPÚBLICA ARGENTINA.
TEL: +54 351 484 8016 – FAX: +54 351 485 0750.
E-mail: soporte@feaselectronica.com.ar
Web: www.feaselectronica.com.ar
DT: Jorge F. Feas, Eng. - PM: 12341991.
The equipment is authorized by A.N.M.A.T. PM-1125-15.
The equipment is sold exclusively to medical professionals and medical
institutions.
AUTHORIZED EU-REPRESENTATIVE
EC REP
Cinterqual, Lda.
Rua Fran Pacheco Nº220, 2º And 2900-374
Setúbal, Portugal
Tel/Fax: +351265238237.
NOTIFIED BODY
PRESAFE
Veritasveien 3,
N-1363 Høvik, Norway.
Postbox 116,
N-1300 Sandvika.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
iii
TABLE OF CONTENTS
SYMBOLSANDREFERENCESLIST........................................................................................................1
NOTESANDWARNINGS.....................................................................................................................2
SCREENMESSAGES.............................................................................................................................8
1.INTRODUCTION............................................................................................................................13
1.1.DEVICEDESCRIPTIONANDINTENDEDUSE...........................................................................................13
1.1.1.DESCRIPTION.................................................................................................................................13
1.1.2.INTENDEDUSE...............................................................................................................................13
1.2.INDICATIONSANDCONTRAINDICATIONS............................................................................................13
1.2.1.DEFIBRILLATIONANDCARDIOVERSION..........................................................................................13
1.2.2.TRANSCUTANEOUSPACINGTHERAPY(OPTIONAL).........................................................................14
1.2.3.PULSEOXIMETER(OPTIONAL)........................................................................................................14
1.3.FRONTPANEL......................................................................................................................................15
1.3.1.MONITORSECTION........................................................................................................................15
1.3.2.DEFIBRILLATORSECTION................................................................................................................15
1.3.3.RECORDERSECTION.......................................................................................................................15
1.3.4.OXIMETRYSECTION.......................................................................................................................16
1.3.5.TRANSCUTANEOUSPACERSECTION...............................................................................................16
1.4.BACKPANEL........................................................................................................................................16
1.5.ACCESSORIES.......................................................................................................................................17
1.6.CONTROLANDINDICATORS................................................................................................................19
1.6.1.CONTROL.......................................................................................................................................19
1.6.2.INDICATORS...................................................................................................................................19
2.INSTALLINGANDTURNINGONTHEDEVICE.................................................................................21
2.1.INSTALLING..........................................................................................................................................21
2.2.CONNECTIONSANDTURNINGONTHEDEVICE....................................................................................22
2.3.TURNOFFTHEEQUIPMENT.................................................................................................................23
2.4.INTERNALBATTERY.............................................................................................................................23
2.4.1.WARNINGMESSAGES.....................................................................................................................23
2.4.2.DESCRIPTION.................................................................................................................................23
2.4.3.CHECKINGTHEBATTERYSTATUS....................................................................................................24
2.5.EXTERNALPOWERSOURCE.................................................................................................................24
3.USEMODE....................................................................................................................................26
3.1.DEFIBRILLATORUSE.............................................................................................................................26
3.1.1.EXTERNALPADDLEPREPARATION..................................................................................................26
3.1.2.EXTERNALPADDLESPOSITIONING..................................................................................................27
3.1.3.MODEOFOPERATING....................................................................................................................27
3.1.3.1.ASYNCHRONICMODE..............................................................................................................................28
3.1.3.2.SYNCHRONICMODE................................................................................................................................28
3.1.4.TIMEOFENERGYCHARGE..............................................................................................................28
3.1.5.USEINADVERSEWEATHERCONDITIONS........................................................................................29
3.2.MONITORUSAGE................................................................................................................................29
3.2.1.SCREENMESSAGES........................................................................................................................29
3.2.2.SETUPMENU.................................................................................................................................29
3.3.ECG......................................................................................................................................................29
3.3.1.WARNINGMESSAGES.....................................................................................................................29
3.3.2.DESCRIPTION.................................................................................................................................30
3.3.3.PLACINGELECTRODES....................................................................................................................30
3.3.3.1.THREEWIREPATIENTECGCABLECONNECTION.......................................................................................30
3.3.3.2.FIVEWIREPATIENTECGCABLECONNECTION...........................................................................................31
3.3.3.3.DISPOSABLEELECTRODEPLACING............................................................................................................31
3.3.4.ECGMENU.....................................................................................................................................31
3.3.4.1.SELECTECGCURVEGAIN.........................................................................................................................31
3.3.4.2.TOSELECTLEADS.....................................................................................................................................31
3.3.4.3.TOSELECTSWEEPSPEED.........................................................................................................................31
3.3.4.4.TOVARYLIMITSOFHEARTRATEALARM..................................................................................................31
3.3.4.5.TOMUTESOUNDALARM.........................................................................................................................32
3.3.4.6.DATEANDTIME.......................................................................................................................................32

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
iv
3.3.4.7.VOLUME..................................................................................................................................................32
3.3.4.8.CONTRAST...............................................................................................................................................32
3.4.RECORDINPAPER................................................................................................................................32
3.4.1.AUTOMATICMODE........................................................................................................................33
3.4.2.MANUALMODE.............................................................................................................................33
3.4.3.INDICATOROFPACEMAKERSPIKEDETECTION................................................................................34
3.5.PACEMAKER........................................................................................................................................35
3.5.1.WARNINGMESSAGES.....................................................................................................................35
3.5.2.WARNINGTRANSCUTANEOUSPACERUSAGE.................................................................................35
3.6.PULSEOXIMETER.................................................................................................................................36
3.6.1.WARNINGMESSAGES.....................................................................................................................36
3.6.2.OXIMETERCONNECTION................................................................................................................37
3.6.3.PATIENTSELECTION.......................................................................................................................37
3.6.4.WARNINGMESSAGESFROMTHEOXIMETER..................................................................................37
3.6.5.CALIBRATION.................................................................................................................................39
3.6.6.MEASUREMENTPRINCIPLES...........................................................................................................40
3.6.7.OXIMETERALARMS........................................................................................................................40
3.7.ALARMSYSTEM...................................................................................................................................41
3.7.1.PHYSIOLOGICALALARMS...............................................................................................................41
3.7.2.TECHNICALALARMS.......................................................................................................................42
4.MAINTAININGTHEEQUIPMENT...................................................................................................43
4.1.GENERALINSPECTION..........................................................................................................................43
4.2.CLEANING............................................................................................................................................43
4.2.1.PADDLECLEANING.........................................................................................................................43
4.2.2.ELECTRODES..................................................................................................................................44
4.2.3.POWERCORD................................................................................................................................44
4.2.4.CLEANINGOFPAPERCHAMBERANDPRINTHEAD...........................................................................44
4.2.5.OXIMETRYSENSORCLEANING........................................................................................................44
4.3.DISINFECTIONMETHODS.....................................................................................................................44
4.4.STERILIZATIONMETHODS....................................................................................................................44
4.5.PAPERREPLACEMENT..........................................................................................................................44
4.6.POWERSELECTOR................................................................................................................................44
4.7.VERIFICATIONOFENERGYDELIVERED.................................................................................................45
4.8.ALARMVERIFICATION.........................................................................................................................46
4.9.FUSECHANGE......................................................................................................................................47
4.10.RECHARGEABLEBATTERY..................................................................................................................47
5.TROUBLESHOTING........................................................................................................................48
6.TECHNICALSPECIFICATIONS.........................................................................................................51
6.1.GENERAL.............................................................................................................................................51
6.2.DEFIBRILLATOR....................................................................................................................................52
6.3.ECG......................................................................................................................................................53
6.4.RECORDER...........................................................................................................................................55
6.5.TRANSCUTANEOUSPACER...................................................................................................................56
6.6.PULSEOXIMETER.................................................................................................................................56
6.7.AUDITORYALARMSIGNALS.................................................................................................................56
6.7.1.AUDITORYALARMSIGNALSOFHIGHPRIORITY...............................................................................57
6.7.2.AUDITORYALARMSIGNALSOFMEDIUMPRIORITY.........................................................................57
6.7.3.AUDITORYALARMSIGNALSOFLOWPRIORITY...............................................................................58
6.8.VISUALALARMSIGNALS......................................................................................................................58
6.8.1.VISUALALARMSIGNALSOFHIGHPRIORITY....................................................................................58
6.8.2.VISUALALARMSIGNALSOFAVERAGEPRIORITY.............................................................................58
6.8.3.VISUALALARMSIGNALOFLOWPRIORITY......................................................................................58
6.9.QRSBEEPSOUND................................................................................................................................58
6.10.CONFIRMATIONSOUNDOFKEYPRESSED..........................................................................................59
6.11.MANUFACTURER’SGUIDANCEANDDECLARATIONREGARDINGELECTROMAGNETIC
COMPATIBILITY....................................................................................................................................60

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 1 of 63
SYMBOLSAND REFERENCES LIST
Attention! Consult accompanying
documents. Defibrillation-proof type CF.
CLASS I Protection against electric shock:
“CLASS I” (with protective earth (ground)). Serial number.
Reference code. Protected against vertical waterfalls witha maximum
inclinationof 15 degrees.
Authorized representative. Battery fully charged.
Line/Battery charge. Low battery.
Date of manufacture. Battery discharged.
Not suitablefor explosive atmosphere. Sound volume.
Connected to line or external 12Vdc. Screencontrast.
Right. Up.
Left. Down.
Protective earth (ground). Warning! High voltage.
Protect from rain. Do not pilemore than 3 boxes.
Fragile. Box orientation.
Charge button. Shock button.
Sync key. Recorder.
Disarmkey. Alarmkey.
Manufacture. Follow the instructions.
Alarm silence. For disposal follow local regulations.
Storage and transport relative
humidity (non-condensing),
maximum and minimum.
Storage and transport temperatu
r
e, maximum and
minimum.
Storage and transport pressure,maximum and
minimum.
Label layout as Annex III.B A.N.M.A.T. 2318/02.
Back label: CE and power supply.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 2 of 63
NOTESAND WARNINGS
WARNING! The followings are descriptions of the general dangers and unsafe usage of the Defibrillator Monitor Mod 3850B-
Biphasic, which can lead to death or severe damage to the user or the equipment.
¾You should read this manual before beginning installation and use of equipment.
¾This equipment is meantto beused by persons trained inprofessional health care.
¾Defibrillator Monitor Mod. 3850B-Biphasic use is limited to a singlepatient at a time.
¾This equipment must be used in conjunction with signs and symptoms of the patient. The equipment is designed to be an aid in
clinical diagnosis.
¾Do not reuse any element disposable or single use. The time limit of use thereof is that indicated by the manufacturer.
¾The expiration date of this equipment is of 5 years from its purchase, when that time is over, dismiss the equipment and it’s
accessories following the local regulations in force.
¾In case of discarding the equipment or one of its accessories, at the end of its useful life, do it according to the local regulations,
normative or lawsin force.
¾In order to take care of the environment, you can send the equipment to feas ELECTRÓNICA for its disposal.
¾RISK OF ELECTRICAL SHOCK, if you remove the equipment’s lead. Do not remove the equipment’s lead. Ask for assistance
from qualified and authorized personnel.
¾There is a risk of Electrical Shock and death. Do not use this equipment if you doubt of the integrity of any cable. Checkthe cables
periodically (disconnect them before) to verify their integrity; paying special attention to cable points close to connectors and
paddles. In case youfind an irregularity, request the part to our Customer’sService.
¾Do not touch the Power Line connectors with wet hands.
¾The Defibrillator Monitor Mod 3850B-Biphasic is designed with covers and plastic handles to minimize the risk of electrical shock.
When it is notpluggedtothelinepower its energy will be providedby the battery, without ground reference.
¾The multi-outlet power extension cord shall not be placed on the floor.
¾The multi-outlet power extension cord should only be usedto power equipmentthat is part of the system.
¾All combinations of medical equipment with non-medical equipment must comply with the total leakage current specified in IEC
60601-1, Cl. 16.
¾When combined instruments, the sum of the leakage currents can be dangerous for both the patient and the operator. If you can’t
determine the leakage current of each team on the specifications of each, technical staff will take measurements to ensure the
installation complies with the requirements of EN 60601-1, Cl. 16. In any case, the user should consult the manufacturer to ensure
that thesum of leakage currents will not jeopardize patient safety.
¾The device is intended to be connected to:
- Installations in medical rooms belonging to Groups 0, 1, 2a and 2b, according to AEA90364-7-710 (IEC 60364-7-710).
- Installations according to AEA90364-7-771, installations in buildings in general, to grounded outlets according to IRAM 2071.
- Mobile units, to the cigarette lighter connector of the vehicle.
¾The equipment must be connected to an approved electrical installation that includes a correct grounding according to the local
legislation in force. Do not use adapters or replace the device’s original cables. If the plug does not match the installation, please
contact our Customer Serviceforthe provisionof a suitable cable.
¾You must make sure that the AC outlet, to which you will plug the device, has a groundling and is in good condition.
¾Do not connect this device toan outlet controlled by a switch on/off.
¾Verify the AC voltage range matches the voltage at which the equipment is to be connected. If does not match, contact feas
ELECTRÓNICA’s Service Customer.
¾Verify the AC frequency indicated on the back panel matches the AC frequency which the device is to be connected. If does not
match, contact feas ELECTRÓNICA’s Service Customer. Do not use the device in those conditions. The ECG curve will be
affectedby noise and will notbe able to useSYNC mode.
¾If you have any doubts about the integrity of the ground, either the cable or the installation of the building, use the device from the
internal battery. In case that the battery is discharge or damage, don't use the equipment.
¾The disconnection of the equipment from the Power Line does not de-energize it, since it has an internal battery, so you must also
set the selector switch to OFF.
¾Caution, leave one end of the power cord accessible so that in the event of an emergency, it is easy to disconnect the equipment
from the Power Line.
¾Do not disconnect power bypullingthe cord. Disconnect the connector firmly grasping.
¾Do not excessively bendthe plug or power cord orplaceheavy objects on them, which could causedamage.
¾Do not immerse the electrical connector in liquids. Thiscan damage the cable or connector by corrosion.
¾RISK OF FIRE AND/OR EXPLOSION: do not use this equipment in the presence of flammable gases (anesthetics, oxygen, etc).
¾Not use or store inflammable substances near the equipment.
¾Avoid installing this equipment in places where liquids can be spilled on it. Avoid direct exposure to splatter, sprayer or air vented
from nebulizers or humidifiers.
¾Do not place containers with water, chemicals or any small metal objects on the equipment.
¾Do not use this equipment under the rain. You have to make sure that the equipment, the cables and paddles are dry before you
start using them.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 3 of 63
¾Do not place the equipment on the patient or where it can fall over the patient. Place it next to the patient where it’s comfortable for
its use.
¾Never attempt to introduce sharp, metallic or other objects into any aperture on the equipment.
CAUTION! The followings are the general descriptions of precautions and unsafe usage that could cause slight injuries, damage or
wrong performance of the equipment.
¾The operation of the equipment can be affected bythepresence of CAT scanner.
¾The operation of the equipment can be affected bythe presence ofportable andmobile RF communications equipment.
¾Do not use thisequipment near Magneticresonance imaging equipment (MR o MRI).
¾The operation of the equipment below the specified amplitudes can cause inaccurate results.
¾The Defibrillator should not be used adjacent or stacked with other equipment, if it is necessary to use it adjacent or stacked with
other equipment, the Defibrillator should be observedto verify its normal functioninginthe configurationin whichitis being used.
¾To attach wires and sensors always use hypoallergenic tape.
¾Do not store the equipment in deposits or between periods of use in places where the sun shine directly on it. Risk of damage to
the cover of the equipment, parts and accessories.
¾Avoid installing this equipment in those places where the sun hits directly.
¾Do not place heavy objects on the equipment.
¾Do not drop the equipment when moving it.
¾Use the equipment on aflat and stable surface.
¾Important!! If using a bracket, make sure that the bracket holds at least twice the weight of the equipment. If you have any
questions, please contact feas ELECTRÓNICA.
¾Do not push the keys of the frontal panel with pushing or slicing elements. This will produce permanent damage to the keypad.
Only push the keys of thefrontal panelwith your fingers. Do not press the buttons with yournails.
ACCESSORIESMESSAGES
WARNINGS
•The proper operation of the equipment and protection against the effects of the discharge of a cardiac defibrillator requires the
use of original accessories intended for this equipment. Only use original accessories provided with the equipment or those
accessories specially indicated for this equipment.
•The use of accessories, transducers and cables other thanthose specified, withtheexceptionof the transducers and cables sold
by the equipment manufacturer as replaceable parts of internal components,may cause an increasein emissionsor a decrease.
•The user shall be responsibleto checkthe compatibility betweenthe accessories used and this medical device.
CAUTIONS
•Do not clean or disinfect the accessory’s cable, accessories, and parts of the equipment or its main body with sodium
hypochlorite, solvents, acids or abrasive products. For cleaning and disinfection of equipment follow the instructions given in this
manual.
•Risk of equipment breakdown. Do not sterilize this equipment or its parts or accessories in autoclave or ethylene oxide. Do not
submerge any part of this equipment in water or other liquids or use abrasive cleaners. Do not spray or spill liquids on the
equipment or its accessories. Do not allow any liquid entering the connectors or other openings of the housing. If you
accidentally spill liquid on the equipment, turn the energy switch OFF (because the equipment has internal battery) and
disconnect it the power line (in case it is connected to the power line), clean it and dry it before reuse. If in doubt about
equipment security, send it to an authorized technical service.
BATTERY MESSAGES
WARNINGS
•The battery must be replaced by an original battery for this equipment. Replacement with another type of battery can result in an
unacceptable risk of temperature rise, fire and/orexplosion of the battery or the equipment.
CAUTIONS
•The equipment has rechargeable battery typemust remainconnected to the power line during periods whenit is not used.
•If the capacity of the internal battery is below 80% the equipment will be able to function from line power or Ext +12Vdc.
•Do not dischargethe battery completely.
•Recharge the battery immediately after it’s been used.
•The internal battery of this equipment cannot be replaced by the user. It has to be replaced by qualified and authorized
personnel.
•In case you replace the battery follow the local instructions to dispose Ni-Mh batteries or send them to feas ELECTRÓNICA
for disposal.
•When the device is stored in warehouse, should be put to charge at least once every 60 days for at least 3hrs at 25ºC ±3ºC
temperature, toprevent battery damage.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 4 of 63
DEFIBRILLATOR MESSAGES
WARNINGS
•Discharging a defibrillator directly to ahealthy person’s chest can be lethal.
•In order to decrease the time pre-shock should follow the specific CPR protocols of the place.
•Neonatal and pediatric defibrillationenergy levels should be set based onthe specificclinical protocols.
•Conscious patients must be anesthetized or sedated before performing synchronized cardioversion.
•Before performing cardioversion, correct synchronism with R wave must be assess.
•When defibrillating the patient be careful to avoid the contact between patient’s body parts (exposed skin, head, arms and legs)
with metallic objects (such as parts of the bed) that might generatenon desired paths for the defibrillation current.
•Rescuers performing chest compressions during external defibrillation are exposed to leakage currents.
•Do not touch the bed, patient’s body, or any equipment connected to the patient during defibrillation. A severe electrical shock can
result.
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for defibrillation since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.
•This equipment is protectedagainst the effects of defibrillation.
•Make sure you know where and how to position the paddles for monitoring and defibrillation. See section External paddles
positioning.
•When defibrillatingwith paddles, use yourthumbsto operate the SHOCK buttons in order to avoid inadvertent operator shock.
•When positioning the paddles onthepatient forenergy discharge, make sure noone isnear or in contact with the patient.
•Avoid spilling conductive paste or gel over hand or inthepaddleshandles as this might cause and electric shock totheoperator.
•Avoid the excess of conductive paste or gel over the patient’s thorax as it could generateand electric path overthepatient’sskin.
•To avoid risk of electrical shock, do not touch thegelled area of thepads while pacing.
•Take special care to keep the paddles pressing firmly on the patient, since a poor contact with the patient may cause interference
(noise) resulting in a false trigger and shocks the patient, as well as cause burns at the shock time.
•Never defibrillate a patient with the paddles wet.
•Never defibrillate a patient on a wet surface.
•More than 10 energy discharges should not be performed during the Test of Energy Delivered; considering that the minimum
waiting time is 1 minute, between discharges.
CAUTIONS
•For safety reasons, after completion of the energy charge and elapsed the “automatic disarm time”, the defibrillator will discharged
the energy internally automatically. By default, the “automatic disarm time” is 60 seconds. For more information on how to
configure the “automatic disarm time” refer to SETUP MENU section.
•Verify that the devices connected to the patient are protected against defibrillation before shock to the patient. If necessary
disconnect the patient from those devices that are not protected against defibrillation so that they are not damaged by theshock.
•It is recommended DO NOT USE Xylocaine to apply a shock.
•Be sure to knowthemethods used to discharge the energy charged in the Defibrillator Monitor Model 3850B.
•Do not discharge the Defibrillator Monitorplacing paddle against paddle or in the paddle support.
•It is suggested to perform the Test of Energy Delivered according to the domestic policy of the Institution Health or at least once a
week.
•Before performing the Test of Energy Delivered, external paddles must be connectedand place on the paddles holder. Perform the
verification with the equipment connected to line power.
ELECTROSURGERY MESSAGES
WARNINGS
•It is recommended to place the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor away from the surgical field in the
case in which it willuse an electrocautery; this is to prevent burns on thepatient’sbody in the area of the electrode.
•The neutral electrode of the electrocautery must have adequate contact with the patient, otherwise it may cause burns to the
patient.
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for electrosurgery since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.
•The use of anelectrocautery it can causeinterference in the operation ofthis device.
CAUTIONS
•Operation of this equipment may be affected by the presence of strong electromagnetic or radio frequency fields such as those
produced by electrocautery.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 5 of 63
ECG MESSAGES
WARNINGS
•Conductive parts of electrodes and associated connectors for applicable parts shall not contact with other conductive parts of
equipment (metal part), includingmetal parts of equipment connected to ground.
•Make sure you knowwhere and how to position the electrodes for monitoring. See section “Placing electrodes”.
•After the delivery of a defibrillator shock, if monitoring is undertaken with a combination of gel and patch/paddle, the ECG may
initially display no signal or asystole, regardless of the true rhythm.
•It is recommended to place the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor away from the surgical field in the
case in which it willuse an electrocautery; this is to prevent burns on thepatient’sbody in the area of the electrode.
•The heart rate may be affected in the presence of arrhythmias. The cardiotachometer uses an algorithm to determine the heart
rate.
•This equipment may reject pacemaker pulses of the following characteristics: with amplitude of ±2mV to ±700mV and a pulse
widthof 0.1ms to 2ms, anyway keepthepatientswith pacemaker underclose surveillance.
•Electrocardiographic monitoring equipment and its accessories shall not be considered life-supporting medical equipment.
•PATIENTS WITH PACEMAKERS. The rate meter may continue to count the pacemaker rate during some occurrences of cardiac
arrest or some arrhythmias. Not based entirely on the rate meter alarms. Keep patients under close surveillance.
•Be careful when making a temporary suspension audible alarm signal (silence), keep the patient under close surveillance at all
times. If thealarm signals are selected, the visual alarm signal will continue to indicate an alarm condition if this occurs.
•Maximum channel height: 21.2mm. Suitable for use as a monitor up to 2 meters away.
•Carefully route the patient cables, extension cables, oximetry sensors and/or pacemaker electrodes to reduce the possibility of
patient entanglement or strangulation.
CAUTIONS
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for defibrillation since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for electrosurgery since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.
•During monitoring, the ECG electrodes should be re-positioned every 48 hours to maintain good signal quality. After 48 hours, the
electrode’s conductive paste or gel begins to dry andthepatient’s skin maybegintochafe.
•For ECG monitoring using hypoallergenic adhesive electrodes. The company recommends 3M ECG electrodes.
PACEMAKER MESSAGES
WARNINGS
•Conductive parts of electrodes and associated connectors for applicable parts shall not contact with other conductive parts of
equipment (metal part), includingmetal parts of equipment connected to ground.
•Make sure you knowwhere and how to position the pacerelectrode for monitoring. See sectionTranscutaneous pacer usage.
•It is recommended to place the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor away from the surgical field in the
case in which it willuse an electrocautery; this is to prevent burns on thepatient’sbody in the area of the electrode.
•Use demand mode whenever possible. Use asynchronous mode when the presence of motion artifacts or other sources of
artifacts, of the ECG, render the R-wave detection is not reliable.
•To change the pacing modeisnecessary that the pacemaker is off.
•When pacing in demand mode is performed, the patient cable must be connected from the patient to the equipment. If you do not
connect the cable patient cannot turn on the pacemaker. If the pacemaker is on and disconnect the patient cable, the pacemaker
will turn off.
•If you will shock the patient, at move the selector switch from the MONITOR position to the selected energy value, the pacemaker
will turn off.
•Carefully route the patient cables, extension cables, oximetry sensors and/or pacemaker electrodes to reduce the possibility of
patient entanglement or strangulation.
•Verify mechanical capture by radial pulse when electrical stimulation (pacing) is applied. Consider the use of sedation or an
analgesic, in case the patient experiences discomfort or pain during the application of electrical stimulation (pacemaker).
CAUTIONS
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for defibrillation since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for electrosurgery since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 6 of 63
•If the pacemaker function is used in demand mode for extended periods of time, may be necessary to apply new ECG electrodes.
OXIMETRY MESSAGES
WARNINGS
•This is a functional measuring cannot be used to evaluate the accuracy of a pulse oximeter probe or a pulse oximeter monitor.
•The pulse oximeter cannot measurethe contribution tototal errorof a probe/monitor system.
•The oximeter is calibrated to displaythe visualizationof functional oxygen saturation.
•Significantdysfunctions of hemoglobin affect the accuracy of the SpO2measurement.
•SpO2measurement can be affected by excessive ambient light. If necessary, cover the sensor area with an opaque material (e.g.
surgical gauze).
•Contrast inks introduced into the bloodstream, such as methylene blue, indocyanine green, indigo carmine and fluorescent, can
affect the accuracy of SpO2reading.
•Any condition that may reduce the blood flow, like the usage of cuff to measure the blood pressure or extreme systemic vascular
resistance might cause an improper result in the SpO2and pulse rate measurements.
•Avoid using the Oximetery sensor in any extremity were a baumanometer or any type of catheter is placed.
•Before placing the Oximetry sensor remove nail polish or fake nails, they might cause mistakes in the SpO2 reading.
•If the extremity is in an elevated position, could compromise venous return and provide lower saturationmeasurements. Therefore,
it is recommended to keep the Oximetry sensor at the height of the heart.
•Do not placetheOximetrysensor across the foot of a pediatric patient or on the foot itself.
•All necessary information regarding the toxicity and/or action on the tissues of the materials with which the patient or anyone else
can contact is indicated in each attachment
•It should be noted that values between 70% and 100% SpO2measured by the pulse oximeter will be within ± 2% of the value
measured by a co-oximeter, due to the statistical distribution.
•If the equipment power is interrupted, when returning energy, the equipment will start with the last configuration set by the
operator, except for the minimum oximetry alarm limit, that if it was off or set to less than 85%, will be adjusted to 85%, by
regulatory requirement, and if hasbeen set tomore than85% will retain the value set by theoperator.
•It is recommended to place the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor away from the surgical field in the
case in which it willuse an electrocautery; this is to prevent burns on thepatient’sbody in the area of the electrode.
•Be careful when making a temporary suspension audible alarm signal (silence), keep the patient under close surveillance at all
times. If thealarm signals are selected, the visual alarm signal will continue to indicate an alarm condition if this occurs.
•Carefully route the patient cables, extension cables, oximetry sensors and/or pacemaker electrodes to reduce the possibility of
patient entanglement or strangulation.
CAUTIONS
•Only use the Oximetrysensor provided with the equipment or those specially indicated for this equipment.
•The user is responsible for ensuring compatibility between the sensor, extension cable and this equipment.
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for defibrillation since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.
•It is not necessary to disconnect the ECG electrodes, Pacemaker electrodes and/or Oximetry sensor for electrosurgery since the
equipment is electrically isolated; although the paddles should not be positioned close to or on the electrodes or metal parts in
contact with the patient, if this is not possible remove the electrodes or metal parts before positioning the paddles.
•When placingthe Oximetry sensor “Y” with tape does not stretch or tightenit. Ifthe tape is too tight can cause inaccurate readings
and blisters on the skin of the patient (the blisters are caused bylack of skin breath, not byheat).
•Replace the Oximetry sensor every 2 hoursto allow the patient’s skin tobreathe.
•The operation of the Oximeter can be affected by the presence of Computed Tomography equipment.
•In the presence of strong electromagnetic fields, the reading of SpO2may not be stable, displaying different values in every
second. The device reading will stabilizeafterthe interference ceases or when the device moves away from the emission source.
•The maximum time of application of the Oximetry sensor isindicated initsownmanual.
•The specific use of the Oximetry sensor concerning: the patient population (e.g. age, weight), body part or tissue type to which it
applies and application (e.g. environment, frequency of use, anatomical place, mobility) is indicated in its own manual.
•Be sure to know where and how to position the Oximetry sensor. Refer to the user manual that accompanies the sensor.
MEASUREMENT PRINCIPLES
The SpO2module measures the SpO2content with a continuous non-invasive method to measuring oxyhemoglobin saturation. The
principle of measurement is determining SpO2under cyclic congestion status of the tissues during pulsation. The method determines
how much light emitted by the sensor light source penetrates the patient’s tissue (finger or ear, for example) and reaches the
receiver. The amount of light penetratingthetissue depends onmany factors, many of them are constant; but oneof them, the blood
flow, varies with time as it is pulsed, therefore, the oxygen saturation of arterial blood can be calculated by measuring light
absorption during pulsation. The pulsation control system provides a pulse waveform and a pulse signal. For the measurement, the
DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 7 of 63
wavelengthof red light is 660nm and 940nm for infrared light. The optical power delivered to the patient is 4mW (milliwatts).
ALARM WARNINGS
•Because the patient is monitored but not attended continuously by an operator, it is for this reason that the alarms must be
configured and adjusted appropriately.
•If an alarm limit is set to “---” (inhibited), the alarm condition for that limit will not be detected neither manifested by the alarm
signals (visual and auditory).
•According to the intended use of the device, the user is considered a medical professional trained in the operation of the
device and knowledge, among others, inthe monitoring and surveillance of vital signs, so any adjustment made alarm limits by
an operator (paramedics or nurse) should be carried out under thedirect supervision and user commandment.
MAINTENANCE MESSAGES
WARNINGS
•Before any cleaning, make sure the device is turned off. REMEMBER: The device has internal battery, so even disconnected
from the AC line, may be turn on. Verify the selection switch is in the OFF position and the display doesn’t contain instructions
and is turned off.
•Risk of explosion or fire! Do not allow spill water or other liquid on the device. Unplug the power cord before cleaning or
disinfecting equipment.
•It is not allowed to modify the equipment.
•Not modify thisequipment without authorizationfrom themanufacturer.
•If you modifythis equipment must perform appropriate tests and inspections to ensure continued safe use of equipment.
CAUTIONS
•Risk of equipment breakdown. Do not sterilizethis equipment or its parts or accessories in autoclave or ethylene oxide. Do not
submerge any part of this equipment in water or other liquids or use abrasive cleaners. Do not spray or spill liquids on the
equipment or its accessories. Do not allow any liquid entering the connectors or other openings of the housing. If you
accidentally spill liquid on the equipment, turn the energy switch OFF (because the equipment has internal battery) and
disconnect it the power line (in case it is connected to the power line), clean it and dry it before reuse. If in doubt about
equipment security, send the same to an authorized technical service.
•Risk of equipment breakdown. No sterilize defibrillation paddles of this device in autoclave or ethylene oxide.
•Do not clean the external cover, the cables or the paddles with abrasive or acid products.
•Do not clean or disinfect accessory cables, accessories and parts of the equipment or the main body thereof with sodium
hypochlorite (bleach water), solvents, acids or abrasive products. For cleaning and disinfection of the equipment and its
accessories, follow the instructions in this manual.
•During storage in warehouses and between uses, respects the conditions of temperature, pressure and humidity as defined in
this manual and periods of recharge the internal battery specified.
•It suggests an annual contrasting against calibrated simulators.
•In case of fuses are damage replace with fuses of the same type and value. If thefailure persists, please contact our Customer
Service.
•This equipment has line fuses in both the neutral pole and Phase Line.
•Neitherthisequipment nor any of its parts are sterile or sterilizable.
•The user shall be responsibleto checkthe compatibility betweenthe accessories used and this medical device.
•When the device is stored in warehouse, should be put to charge at least once every 60 days for at least 3hrs at 25ºC ±3ºC
temperature, toprevent battery damage.
DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 8 of 63
Screen visualization
The data visualized in the screen are updated once every second, same for SpO2and pulse rate. The visualized data is the measured
values; they are not averaged andare not perform any other process.
The alarm gets activated at the first pulse rate or SpO2 value, out of range, it might have one second delayed to generate and display
the alarm signal.
Calibration
An annual contrasting the equipment withcalibrated simulators issuggested.
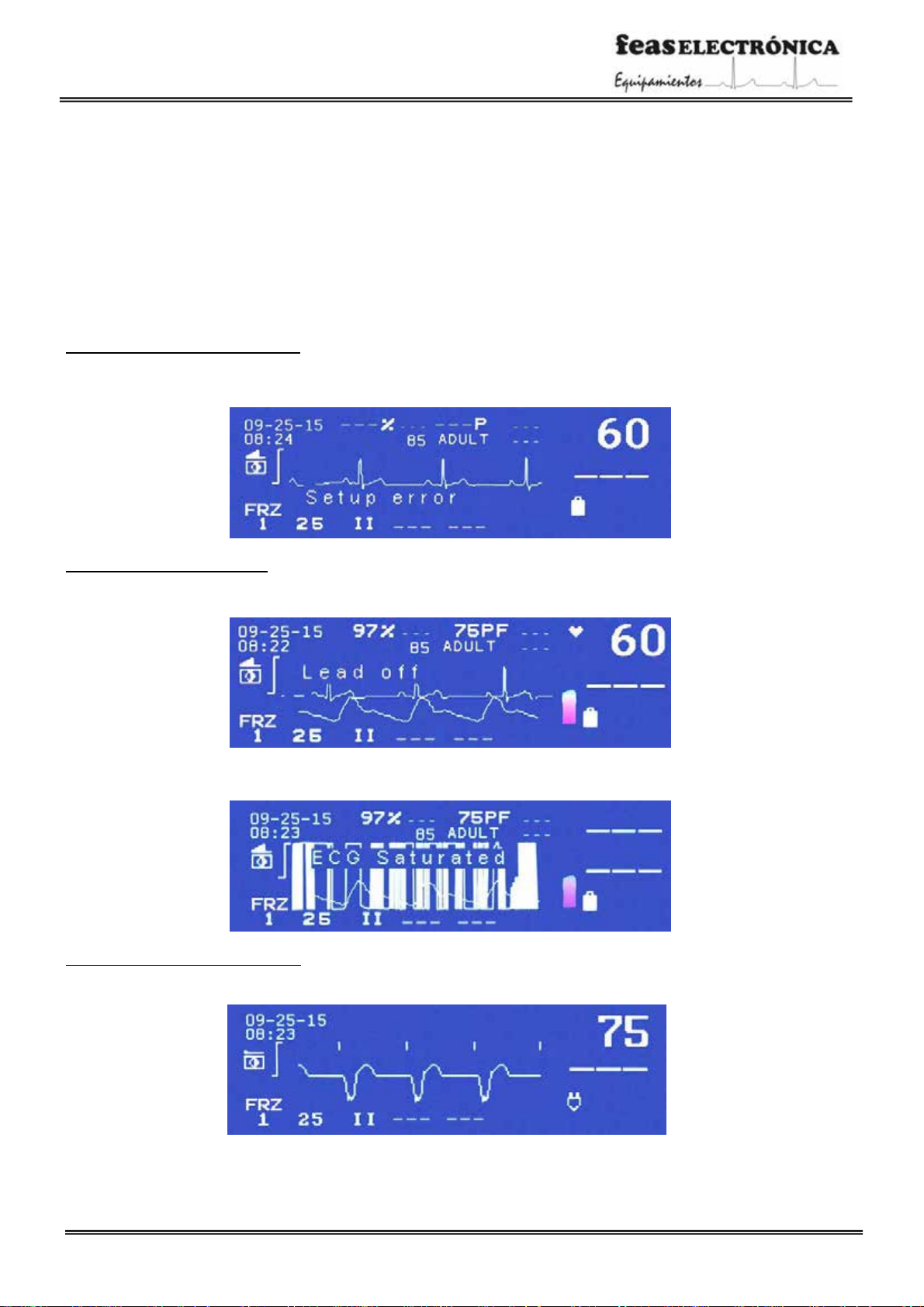
SCREEN MESSAGES
Warning messages from Configuration
“Setup error” This message is displayed when, turning on the device, is located that there are differences between the stored
configurationof factory and the current configuration.
Warningmessages from the ECG
“Lead off” This message is shown when one of the ECG electrodes looses connection with the patient. When this message is
displayed can be selected onlytheleads DI, DII and DIII.
“ECG Saturated (Override)” This message is displayed when, for some reason, ECG channels receive more than ±5mV signal at its
inputs, making inoperative ECG monitoring (Paddles or Patient ECG Cable). This can happen during defibrillation.
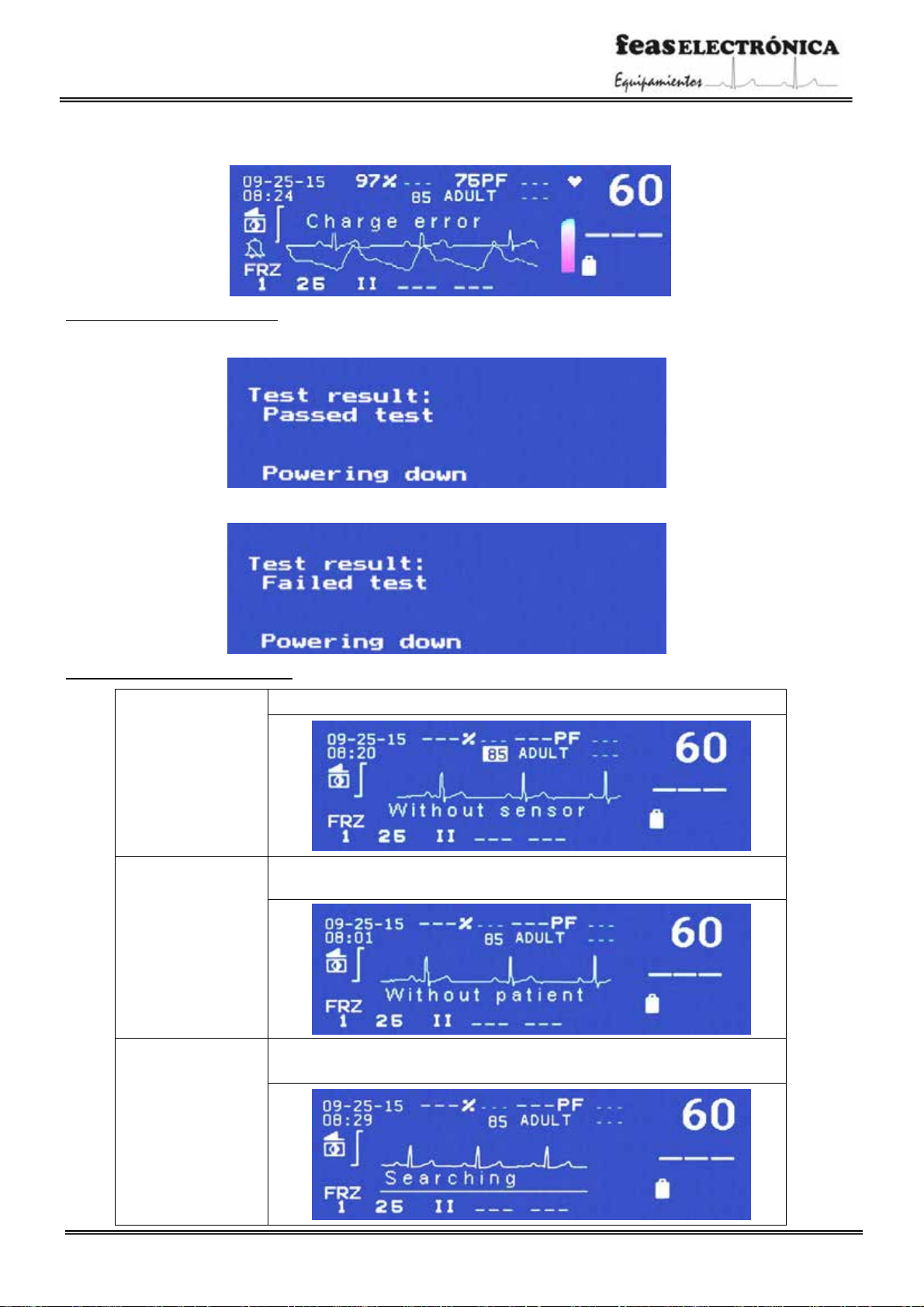
Indicator of pacemaker spike detection
The following figure shows the indication of the presence of pacemaker’s spikes onscreen:
DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 9 of 63
“Charge error” This message is shown when, once the charging command is started, for some reason and after a certain time the
charged energy does not reach the chosen energy value.
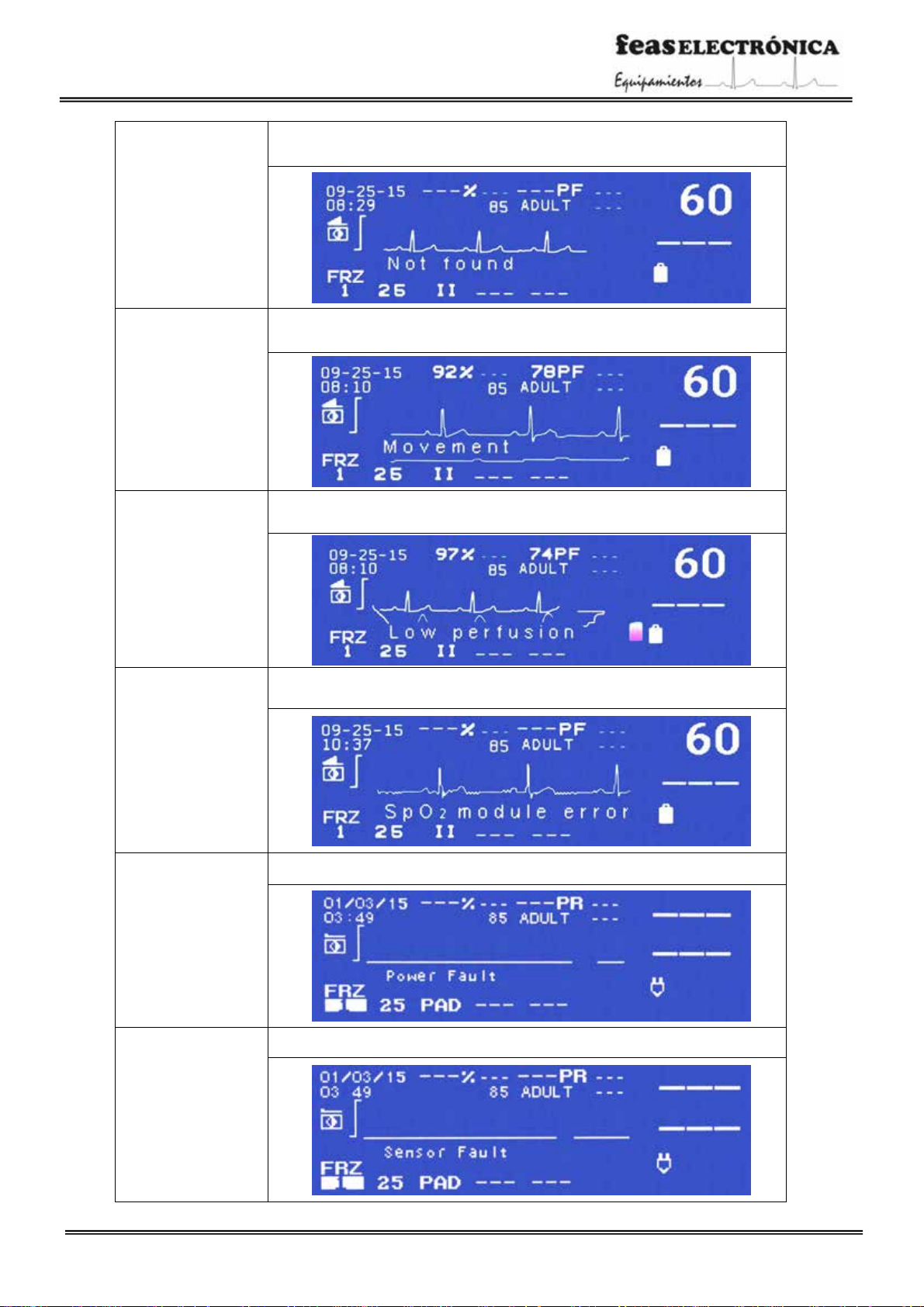
Test messages of energy delivered
“Passed test”: This message is displayed upon completion of theenergy test in the event of a successful outcome.
“Failed test”: This message is displayed upon completion ofthe energy test in case of afailed result.
Warningmessages from the oximeter
Without sensor
This message will be displayed while the Oximetry sensor is not plugged to the device.
Without patient
Once connected the Oximetry sensor, thismessage will be displayed while the sensoris
not placed to the patient.
Searching
After placing the sensor on the patient, the message “Searching” is displayed while the
oximeter detects pulse.
DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 10 of 63
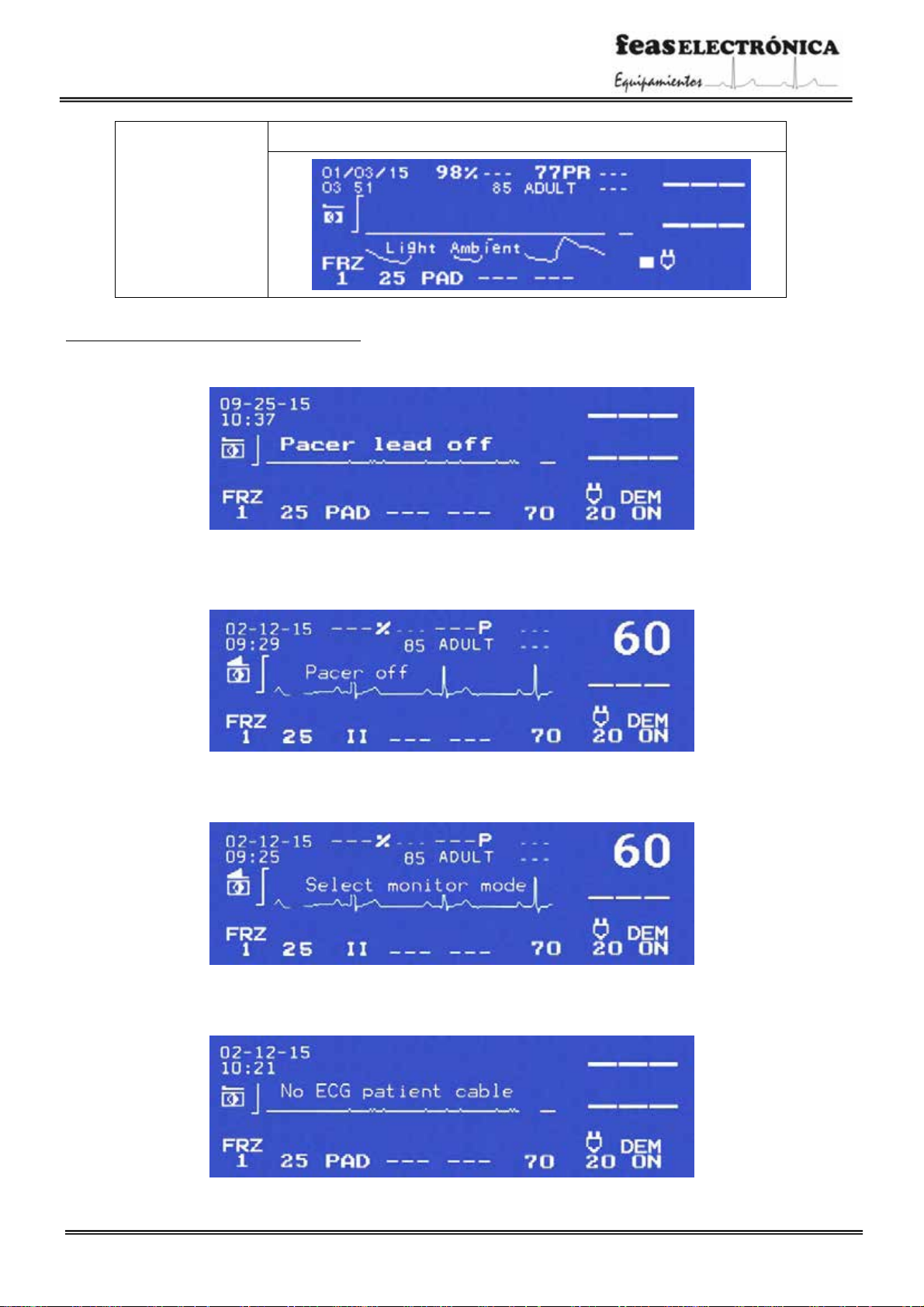
Not found
If pulse is not found after a long period of time, the message “Not found” will be
displayed.
Movement
Once connected the Oximetry sensor andif the device detects patient movement, this
message will be displayed.
Low perfusion
In the presence of a weak signal or low perfusion will display the message “Low
perfusion”.
SpO2module error
If there is no communication with the Oximeter module the message “SpO2module
error” will be displayed.
Power fault
Indicates a power supply failure in the Oximeter module.
Sensor fault
Indicatesthat there is a problem withthe Oximetry sensor.
DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 11 of 63
Interference by
ambient light
When interferenceis detected by ambient light, the message "Light Ambient".
Warning messages Transcutaneous Pacer (TPM)
“Pacer lead off” Thismessageis displayed when an electrode pacer loses contact with the patient and pacerpulse is not applied.
“Pacer Off” This message is displayed when the pacemaker is on and the selector switch moves from position “MONITOR” to the “2
Joules” position, or when the pacemaker is on and mode demand (DEM), and the patient cable is disconnected from the equipment.
“Select monitor mode” This message is displayed when you try to turn on the pacemaker, regardless of the selected mode, and the
selector switch isin aposition of energy selection.
“No ECG patient cable” This message is displayed when you try to turn on the pacemaker in demand mode (DEM) and patient cable is
not connected to the equipment.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 12 of 63
Warning messages RECORDER module
The error message will continue aslongas the cause orcauses that generate it.
Warning messages BATTERY
When the device’s battery is fully discharged and the device is about to power down, the screen will display messages “Battery
discharged” and “Powering down” (see figure below), and in 120 seconds the device automatically turns off. These messages are
accompanied with auditory alarms signal of high priority as described in section “AUDITORY ALARM SIGNALS OF HIGH PRIORITY”.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 13 of 63
1. INTRODUCTION
This user’s manual provides instructions for the installation, operation and basic maintenance, also for safe and appropriate use of the
DEFIBRILLATOR MONITOR 3850B-Biphasic.
BEFORE YOU START USING THE DEVICE, READ THE MANUAL AND GET FAMILIAR WITH its CONTENT.
1.1.DEVICE DESCRIPTION AND INTENDED USE
1.1.1. Description
The model 3850B-Biphasic of feas ELECTRÓNICA is an external defibrillator monitor of rectilinear biphasic waveform capable of
delivering up to 200Joules of energy, selectable by steps; it may be used in asynchronous or synchronous mode to perform
synchronized cardioversion by using the R-wave of the patient ECG as a timing reference. The manual defibrillation implies that the
operator must set the energy,initiate charge and press shockbutton. The energy delivery ismadethrough externalpaddles.
It also allows ECG monitoring, with measure of Heart Rate, through paddles, or through 5 or 3 wires patient ECG cable allowing the
selection of I, II, III, aVR, aVL, aVF and C leads depending on the cable. Optional pulse oximetry (SpO2) monitoring is also available,
plotting plethysmographic curve and a bar graph indicating the pulse amplitude, indicating SpO2and pulse rate values on display. Both,
ECG andSpO2, allows alarms settings of heart rate, SpO2and pulse rate.
It is portable, powered byan internal rechargeable battery, by acline 90Vac - 240Vac, 50Hz or 60Hz, or an external supply of 12Vdc.
Optional transcutaneous pacer is available, to apply temporarily pacing a patient’s heart in external non-invasive way, in fixed mode
(asynchronous) or demand (synchronous) through electrodes.
A thermal recorder is also optionally available. Two real time curves can be registered: an ECG lead and the plethysmographic curve,
optionally. The record can be initialized in manual mode by the operator when decided or in automatic mode during energy charge and
energy shock.
The optional parameters combination is detailed in the next table:
Ref. Description
14634 DEFIBRILLATORMONITOR WITHINTERNAL BATTERY 3850B BIPHASIC.
14635 DEFIBRILLATORMONITOR WITH INTERNALBATTERY AND RECORDER 3850B BIPHASIC/R.
14636 DEFIBRILLATORMONITOR WITH INTERNALBATTE
R
Y ANDPACER 3850B BIPHASI
C
/TPM.
14637 DEFIBRILLATORMONITOR WITH INTERNALBATTERY, RECORDER AND PACER3850B BIPHASIC/R/TPM.
14638 DEFIBRILLATORMONITOR WITH INTERNAL BATTERY AND SpO23850B BIPHASIC/SpO2.
14639 DEFIBRILLATORMONITOR WITHINTERNALBATTERY, RECORDER AND SpO23850B BIPHASIC/R/SpO2.
14640 DEFIBRILLATORMONITOR WITH INTERNAL BATTERY, PACER AND SpO23850B BIPHASI
C
/TPM/SpO2.
14641 DEFIBRILLATORMONITOR WITHINTERNALBATTERY, RECORDER, PACERAND SpO23850B BIPHASIC/R/TPM/SpO2.
1.1.2. Intended Use
Place: It is intended to be used in a great varietyof clinical/hospital ambient such as Intensive Care, Shock Rooms, Coronary Care Units,
OperatingRooms, Emergency Room, HospitalWards and also Out-Of-Hospital or in mobile units suchas ambulances.
Operator/User:To be used only by qualified medical staff trained in the operation of the equipment and expertise in advanced cardiac life
support, procedures defibrillation, cardioversion, cardiac pacing and monitoring vital signs. It can also be used by paramedics or nurses
under the direct supervision and order of a physician.
Patient: It is intended for used in patient neonates, pediatric, adults and senior adults; victims of cardiac arrest, ventricular fibrillation,
ventricular tachycardia, supraventricular tachycardia, atrial fibrillation, bradycardia or other fatal arrhythmias.
1.2. INDICATIONS AND CONTRAINDICATIONS
1.2.1. Defibrillation and Cardioversion
Indications:
Asynchronous defibrillation is the initial treatment for ventricular fibrillation and ventricular tachycardia in patients who are pulse less,
unconscious and not breathing on their own. The synchronous fibrillation or cardioversion is indicated for termination of atrial fibrillation,
atrial flutter or atrial tachycardia.
Contraindications:
Asynchronous defibrillationtherapy iscontraindicated in patients that exhibit one or anycombination of thefollowing:
•Responsiveness.
•Spontaneous breathing.
•Palpable pulse.

DEFIBRILLATOR MONITOR
Mod. 3850B-BIPHASIC - USER MANUAL
Page 14 of 63
Inappropriate defibrillation or cardioversion of a patient (e.g. without malignant arrhythmia) can cause ventricular fibrillation, asystole or
other dangerous arrhythmias.
The defibrillation asystole may inhibit recovery of the heart’s natural pacemaker and completely eliminate any possibility of recovery.
Energy should not be applied routinely during asystole.
The incorrect application of the paddles or gel pad in defibrillation can lead to inefficacy and produce burns, particularly when multiple
shocks are delivered.
Erythema or hyperemia ofthe skin under the paddles can often becaused; this redness shoulddecrease in term of 72 hours.
1.2.2. Transcutaneous pacing therapy (optional)
The Biphasic Defibrillator Monitor 3850B with TPM allows transcutaneous electrical stimulation to the heart by delivering a monophasic
stimulus in demand mode or in asynchronous mode. This stimulus is intended to cause cardiac depolarization and myocardial
contraction. The medical care provider selects the stimulus current and rate settings. The energy is delivered through electrodes applied
to the patient’s bare chest.
In demand mode, thepacemaker delivers pulses onlywhenthe patient's heart rate islowerthanthe selectedpacing rate.
In asynchronous mode, the pacemaker delivers pulses at the selected frequency.
Indications:
Non-invasive pacing is one method of treating patients with symptomatic bradycardia or hemodynamically unstable, particularly in
patients who do not respond to drug therapy.
Hemodynamically significant bradyarrhythmias requiring temporary pacemaker are due to various types of AV block, sinus node
dysfunction or bifascicular block symptomatic.
The transcutaneous cardiac pacing is useful in reversible or transient conditions or when it is not possible transvenous cardiac pacing. It
can also be helpful in patientswith asystole, if performed early.
Contraindications:
Non-invasive electric pacing is contraindicated in the treatment of ventricular fibrillation, it not respond to electrical stimulation and
requires immediate defibrillation. For that reason, the patient arrhythmia should be immediate determinate to apply the correct treatment.
If thepatient is sufferingventricular fibrillation andthe defibrillationwas successful butasystole isproduced, pacing should be applied.
Electrical stimulation is contraindicated in patients with asystole by cardiac arrest, especially if the resuscitation were delayed more than
20 minutes, by the poor performance of these patients.
Ventricular or supraventricular tachycardia could be interrupted with pacing, but synchronized cardioversion is quicker and safer during
emergency to circulatory collapse. An electromechanical dissociation can occurs after a prolonged cardiac arrest; in this case effective
ECG response without mechanical contractions can be produced by pacing, whereby another treatment is required.
Undesirable repetitive responses, tachycardia, fibrillationwith generalized hypoxia, myocardial ischemia, cardiac drugtoxicity, electrolyte
imbalanceor otherheart diseases can becaused by electricpacing.
Stimulation by anymethodtendsto inhibit the intrinsic rhythm.Abrupt rate, especially in high heart rates, may cause ventricular stop.
Non-invasive Temporary Pacing may cause discomfort of varying intensity, which can sometimes be serious and oppose the continued
use in conscious patients.
Similarly, inevitable muscle contraction can be problematic in very ill patients and may limit continuous use to a few hours.
It can often cause erythema or hyperemia of the skin under the electrodes.
The non-invasive pacing in the presence of severe hypothermia may be contraindicated; the bradycardia in these patients is a
physiological phenomenon.
1.2.3. Pulse Oximeter (optional)
Indications:
SpO2monitoring is indicated for use when it is beneficial to assess a patient’s oxygen saturation level. It is indicated for use in any
patient who isat risk of developing hypoxemia.
Contraindications:
There are no knowncontraindicationsforSpO2monitoring through a Pulse Oximeter.
Table of contents
Popular Medical Equipment manuals by other brands

Braun
Braun Aesculap MINOP PaediScope Instructions for use/Technical description

TATA Motors
TATA Motors CHECK ExpressPCR user guide
OrthoSensor
OrthoSensor Knee Balancer user guide
Transcend
Transcend 3 miniCPAP quick guide
Respironics
Respironics bilichek user manual

Forge Medical
Forge Medical VasoStat Instructions for use