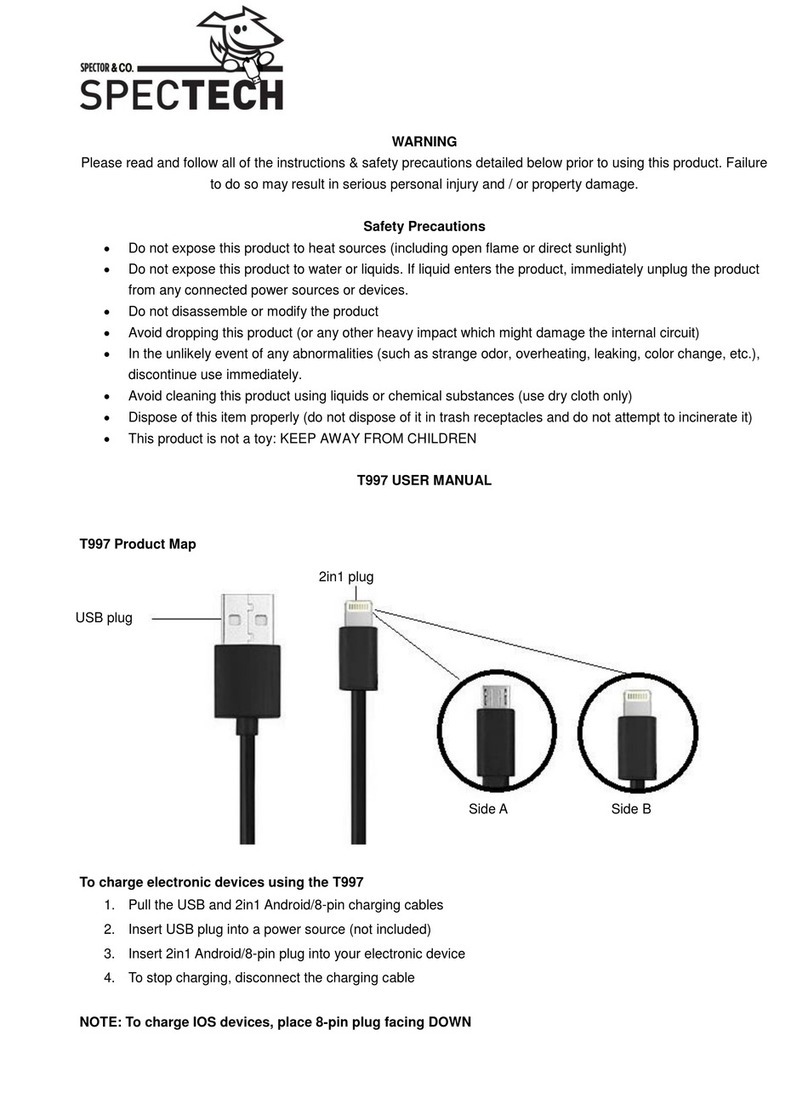
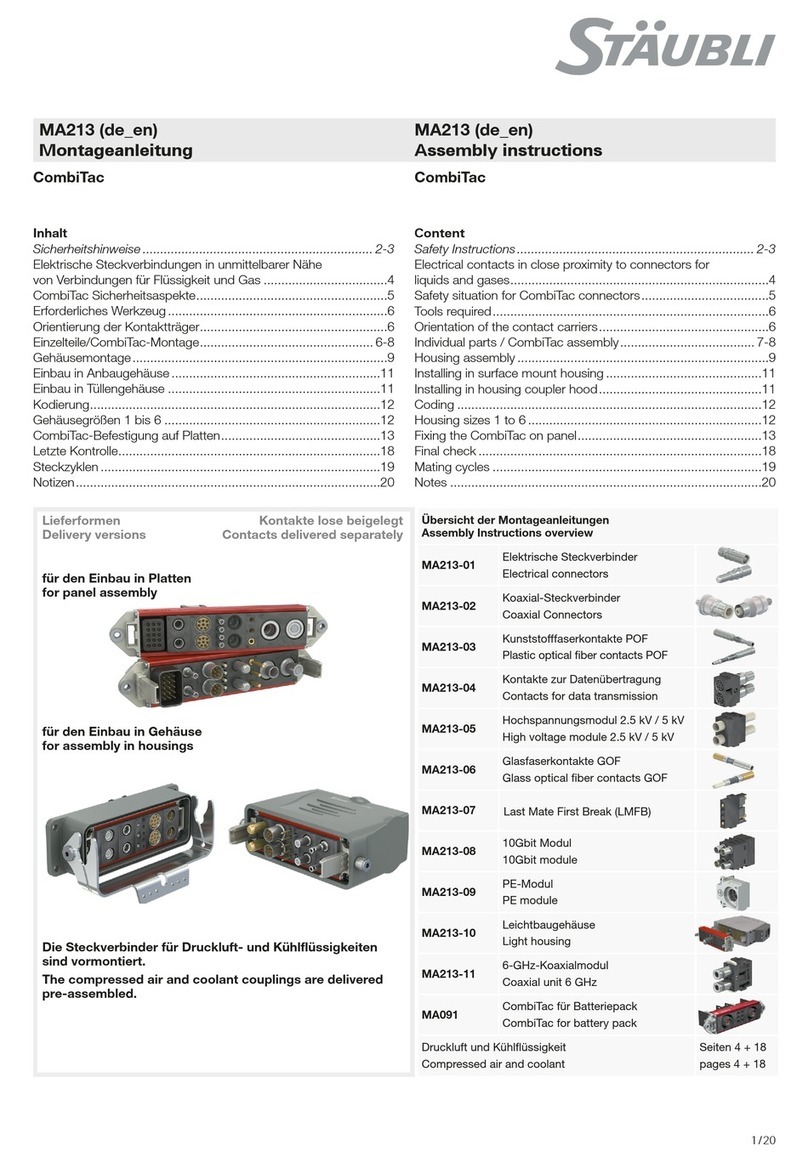
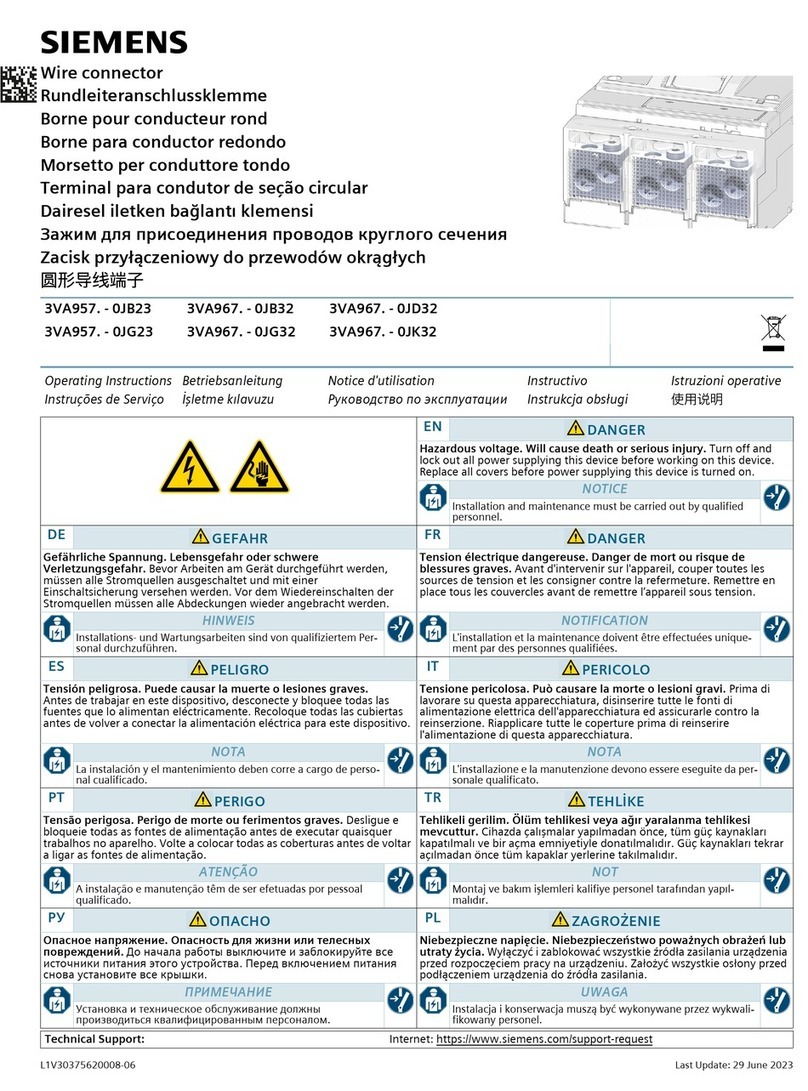
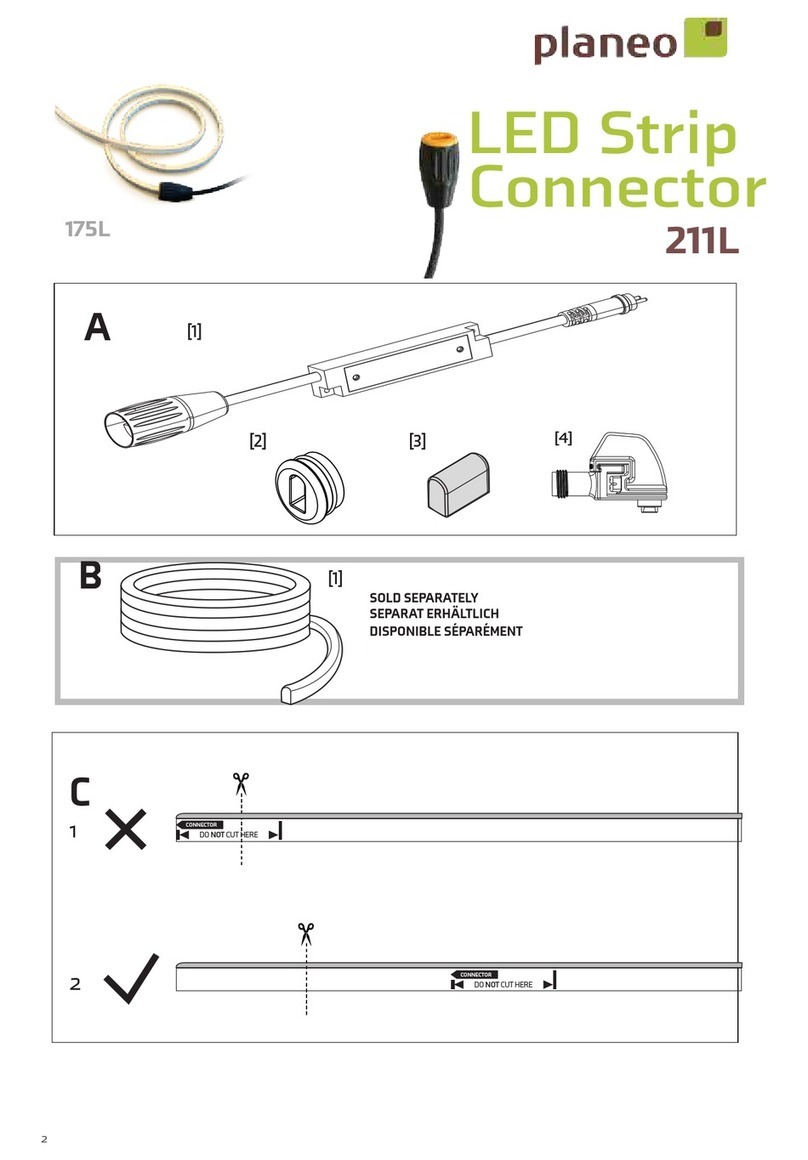
FHC microTargeting C0231 Manual

microTargeting™ Electrode Lead Cable
Directions For Use
L011-85-05 (Rev B0, 2019-06-26)
Contains directions for the following products:
Lead Cables: C0230, C0231, C0232
FHC, Inc.
1201 Main Street
Bowdoin, ME 04287 USA
Fax: +1-207-666-8292
FHC Europe
(TERMOBIT PROD srl)
42A Barbu Vacarescu Str, 3rd Fl
Bucharest 020281Sector 2
Romania
24 hour technical service:
1-800-326-2905 (US & Can)
+1-207-666-8190
FHC Latin America
Calle 6 Sur Cra 43 A-200
Edificio LUGO Oficina 1406
Medellín-Colombia
www.fh-co.com

Page 2 of 4 L011-85-05 Rev B0 2019-06-26
Indications for use
The microTargeting™ Guideline 4000™ 5.0 is intended to record and stimulate electrophysiological
activity, as well as aid in the accurate placement of electrodes and other instruments.
Intended use
The Guideline 5 System is intended to be used by a neurosurgeon, neurologist or clinical
neurophysiologist to accurately position depth electrodes during functional neurosurgical
procedures.
WARNING/Caution, Consult instructions for
important cautionary information
Consult instructions for use
In reference to“Rx Only” symbol; this applies to USA
audiences only
Caution - Federal law (USA) restricts this device to
sale by or on order of a physician
Indicates catalog number
Indicates the batch code
Indicates the date after which the medical device is
not to be used
Medical device that should not be used if the
package has been damaged or opened
Indicates a medical device that is not to be
resterilized
Do not re-use; intended for one use on a single
patient, during a single procedure
Medical device manufacturer, as defined in EU
Directives 90/385/EEC, 93/42/EEC and 98/79/EC.
Telephone number
Authorized Representative in the European
Community
European Conformity. This device fully complies with
MDD Directive 93/42/EEC and legal responsibilities as
a manufacturer are with FHC, Inc., 1201 Main Street,
Bowdoin, ME 04287 USA.
Indicates a medical device that has been sterilized
using ethylene oxide.
Indicates the temperature limits to which the medical
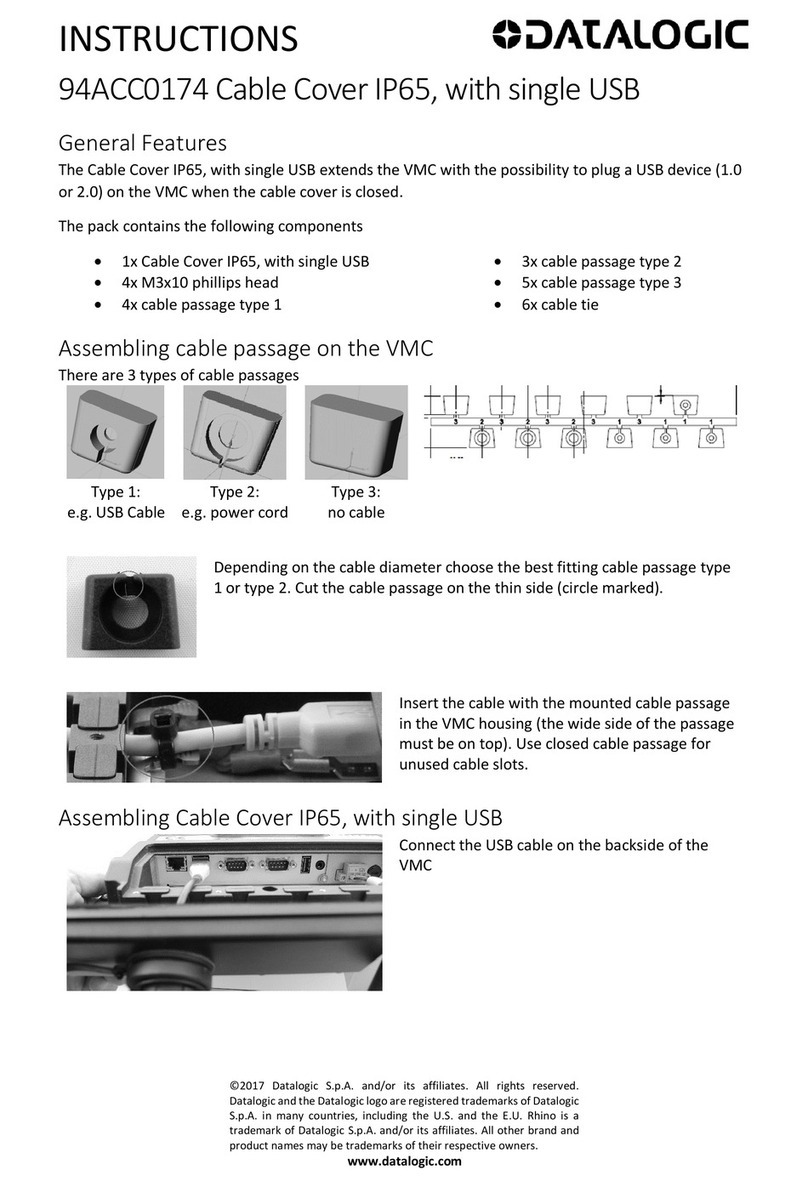
device can be safely exposed
Indicates the range of humidity to which the medical
device can be safely exposed
Symbol Key
Warnings and Cautions
Rx only CAUTION: Federal law (USA) restricts this device to sale by or on the order of a
physician.
WARNING: Sterile Medical Device – Do NOT resterilize.
WARNING: Do not reuse; reusing single-use medical devices could lead to serious patient
injury.
WARNING: Do not use the contents if there is any evidence of damage to the package or
package seal that could compromise sterility.
WARNING: Remove patient leads if engaging defibrillation
WARNING: Disconnect all patient connections when performing system self-test
WARNING: Do not connect the electrode cables to earth.
WARNING: Electrodes and electrode cables should be connected one at a time. Be careful to
assign electrode tracks and electrode contacts correctly. See L011-85-01 for more information
regarding track assignments.
WARNING: Route electrode lead cables carefully to avoid a tripping hazard or possible
contamination of the sterile field.
0°C
(32°F)
40°C
(104°F)
0%
95%

Page 3 of 4L011-85-05 Rev B0 2019-06-26
Handling and Storage
Storage: Store the microTargeting™ Electrode Lead Cable at normal temperatures between 0°C
(32°F) and 40°C (104°F). Do not expose to relative humidity of more than 95%.
Specications
C0230 – 3m Patient Lead
C0231 – 1.5m Patient Lead
C0232 – 1.5m Patient Lead (Special Use)
Note: C0232 is cleared for use in the US only.
Length: 3 meters (C0230) / 1.5 meters (C0231,
C0232)
For Single Use Only, do not re-sterilize
Pre-sterile, sterilization method – EtO
Minimum bend radius: 2cm
Configuration: Dual, shielded Coaxial, 2.5mm OD
Interface Connector: proprietary, 6-pin
Electrode Connectors:
• Color coded, red - macro, blk - micro
• Gold plated (C0230, C0231)
• Alligator Clips (C0232)
• 0.9mm ID x 3.5mm dp
• Insertion/Extraction force: <500g
Reference Connector: Green, mini-alligator clip
Color Coded Flags: white, blue, green, red, yellow
Instructions for Use
The electrode lead cables are designed for use with the microTargeting™ Guideline 4000™ 5.0. For
instructions on operating the Guideline system, refer to the Guideline 4000™ 5.0 Directions for Use,
L011-85. The procedure below commences once the microelectrodes have been inserted.
Connecting an electrode to the Guideline 5 System is a two-step process: connect the
Patient Lead from the electrode to the UE Interface and ‘map’ that connection in the Guideline
application. While these steps may be performed in any order, to avoid the risk of making an
error, they should both be completed for every electrode before moving on to the next one.
Connecting the Patient Lead: Maintaining sterile protocols, peel open the Patient Lead pouch.
Connect the lead contacts to the electrode as shown: black to black for the microelectrode
contact, red to gray/red for the macro-electrode contact and connect the patient reference
alligator clip to the insertion tube or any other suitable patient reference source. Being careful
not to touch the non-sterile UE Interface (or handing off to a non-sterile assistant), plug the
opposite end of the lead into the proper channel of the Interface. It will be the one with the
green status LED.
* Setup may vary for Special Use cables depending on electrode. Place the patient leads on the appropriate section of the
electrode model being used.
C0230/C0231 Setup
C0232 Setup*

Page 4 of 4 L011-85-05 Rev B0 2019-06-26
Repeat the above procedure for all electrodes that will be used during the recording pass. For
an additional level of assurance, colored stickers are provided with the Patient Leads. Selecting
a different color for every Patient Lead used, attach a colored sticker near both ends of the
lead. These color codes can be matched to the colors used within the Guideline Application to
represent that channel.
Associating a color, in this way, with each channel/track/electrode can be very helpful in
keeping straight which channel is which throughout the procedure.
When the MER procedure is complete, remove the lead cable from the electrode and Guideline
5 UE Interface.
Dispose of according to hospital protocol.
Recommendations
Do not apply bends in lead cable. Failure to keep lead cable as straight as possible may degrade
noise immunity.
For C0230/C0231 Configuration
This manual suits for next models
2
Table of contents