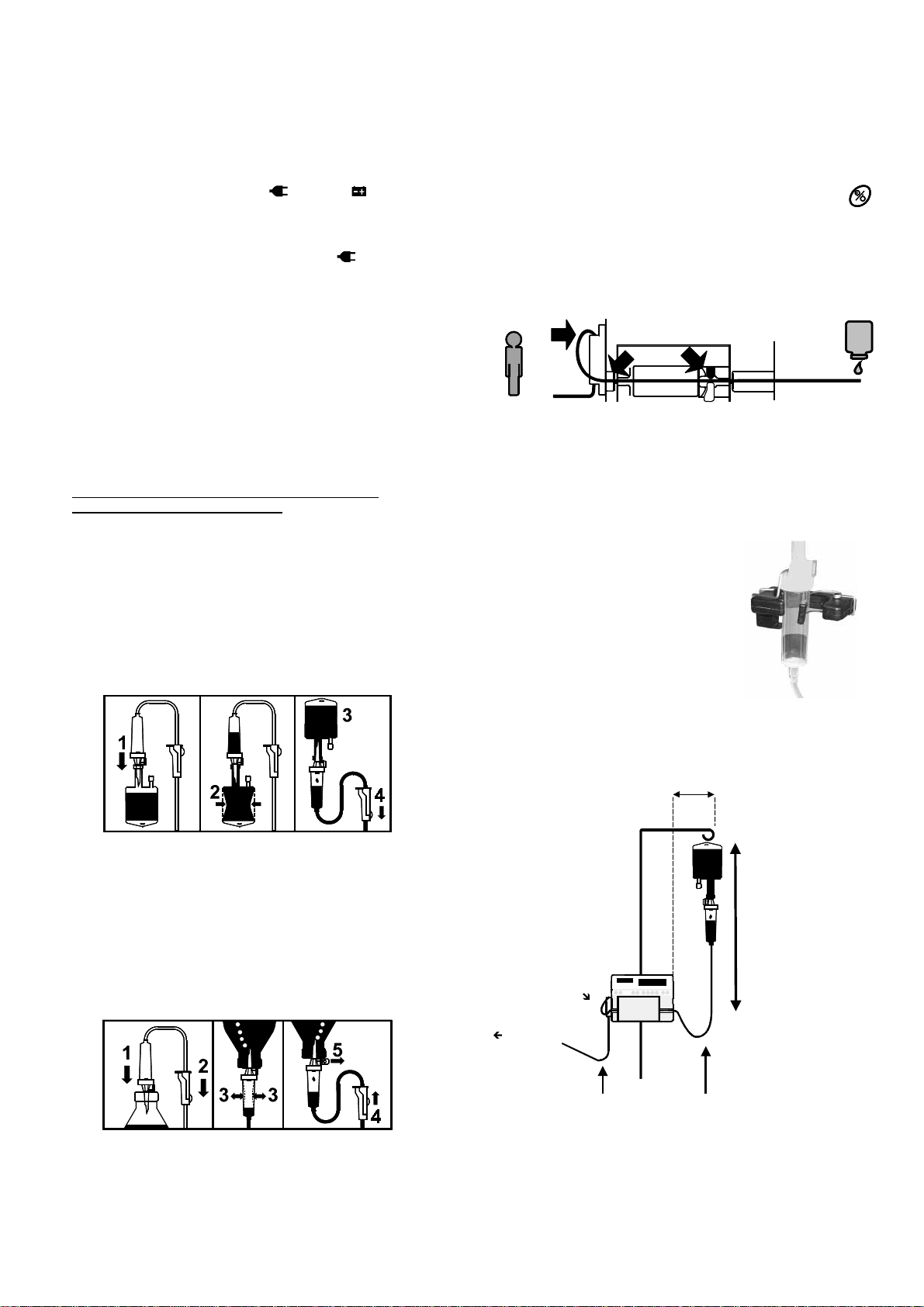
NU OPTIMA PT IS - 1273 rev 0 – 03/12/02
- 6 -
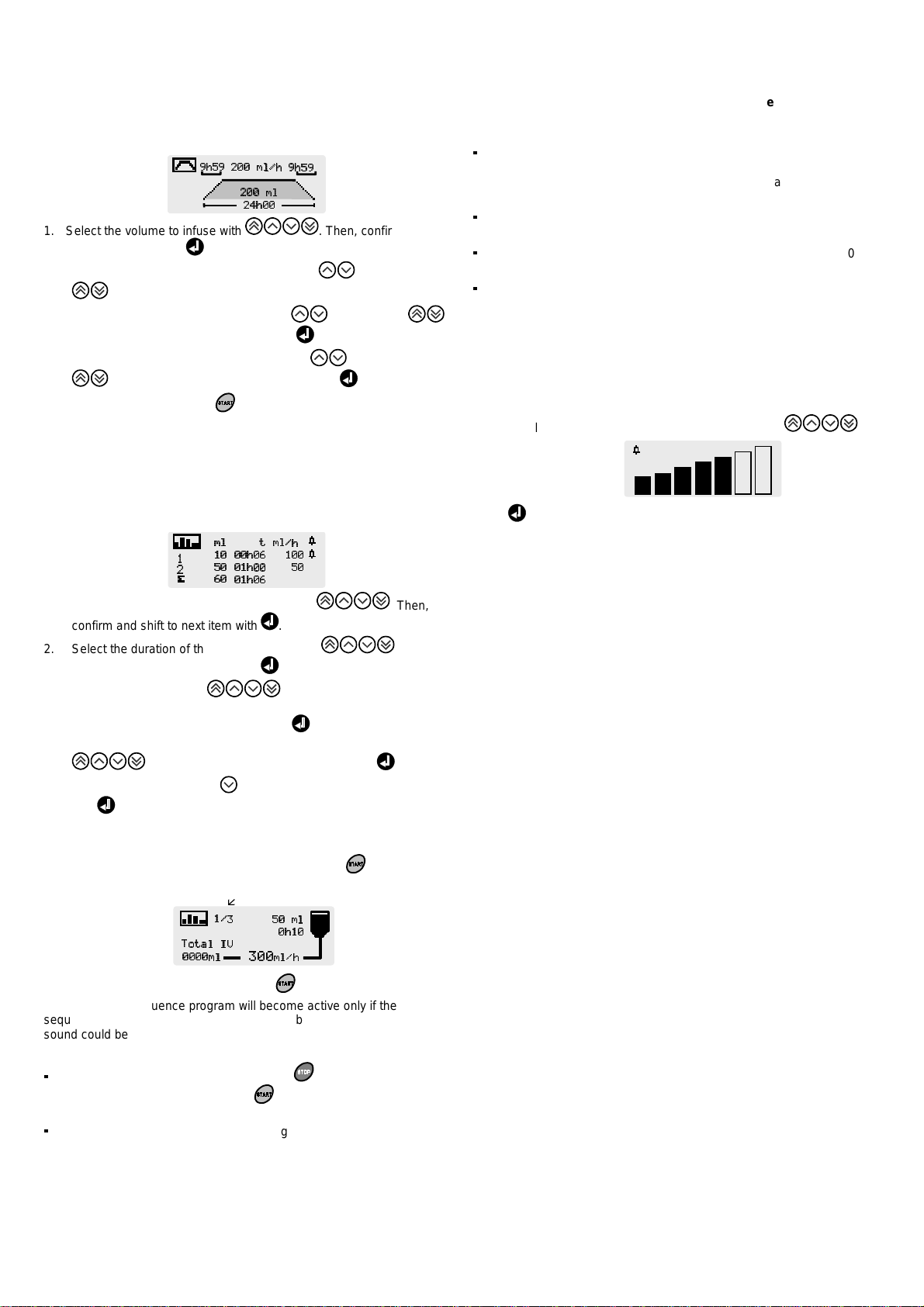
2.9. Ramp up/Ramp down
By setting ramp up and ramp down durations, the pump will
automatically increase the flow rate to reach in ten intermediate
steps the plateau flow rate and, at the end of the infusion plateau,
decrease the flow rate down to zero.
K
K P
POK K
OK K
P
PO
O
K
K
1. Select the volume to infuse with . Then, confirm and
shift to next item with .
2. Select total infusion duration in minutes with , in hours with
. The sustaining flow rate is then automatically calculated.
3. Select ramp-up duration in minutes with , in hours with .
Then, confirm and shift to next item with .
4. Select ramp-down duration in minutes with , in hours with
. Then, confirm and shift to next item with .
5. Start infusion by pressing .
2.10. Sequential programming
From 1 up to 20 infusion sequences can be defined in volume to
infuse and rate of infusion. Pause periods (Stop) or KVO periods
could also be defined in the sequence program:
PO
PO
W
W
K
K
K
K
K
K
PO
PO
K
K
1. Select the volume of the first sequence with . Then,
confirm and shift to next item with .
2. Select the duration of the first sequence with . Then
confirm and shift to next item with .
3. Select the flow rate with . The infusion duration is
automatically calculated and readjusted according to displayed flow
rate. Confirm and shift to next item with .
4. Activate or de-activate beep at the end of the sequence with
. Then, confirm and shift to next item with .
5. Select next sequence with , and shift to next volume to infuse
with .
6. Set new sequence(s) in the same way.
7. End last sequence by selecting “end” as last volume to infuse.
8. Check programming sequences and confirm with key.
P
PO
O
PO
POK
K
P
PO
O
K
K
Number of pending sequence / out of total
Ë
number of sequence
7
7RWD
RWDO
O,
,9
9
9. Start sequential infusion by pressing .
Changes in the sequence program will become active only if the
sequences are re-started or have not already been reached. A beep
sound could be activated at each end of a sequence.
Notes :
To modify the ongoing sequence, press , change the ongoing
sequence parameters and press key to validate. The sequential
program is not modified.
If sequence program is modified during sequential infusion, only
forthcoming sequences will be modified.
2.11. Micro Infusion Flow Rate
When the micro infusion flow rate is activated (see Configuration
menu), a decimal digit is displayed both for flow rate and for
volume.
Flow rate range : from 0.1 ml/h to 100 ml/h, 0.1 ml/h increment.
In order to insure a high flow rate accuracy, the lower limit is set at
0.5 ml/h (see Precaution to be taken before use page 15). For a
setting as of 0.1 ml/h, contact our After-Sales Service Department.
Bolus Flow rate range : from 0.1 ml/h to 300 ml/h, 0.1 ml/h
increment from 0.1 to 100 ml/h, 1 ml/h increment above 100 ml/h.
Volume limit range for primary infusion : 0.1 ml to 1000 ml, 0.1
ml increment.
Volume limit for secondary infusion : 0.1 ml to 1000 ml, 0.1 ml
increment from 0.1 to 100 ml; 1 ml increment, above 100 ml.
Note: volume is displayed at ±0.1 ml.
2.12. Sound level
The sound level can be adjusted with the selection keys .
Press to confirm.