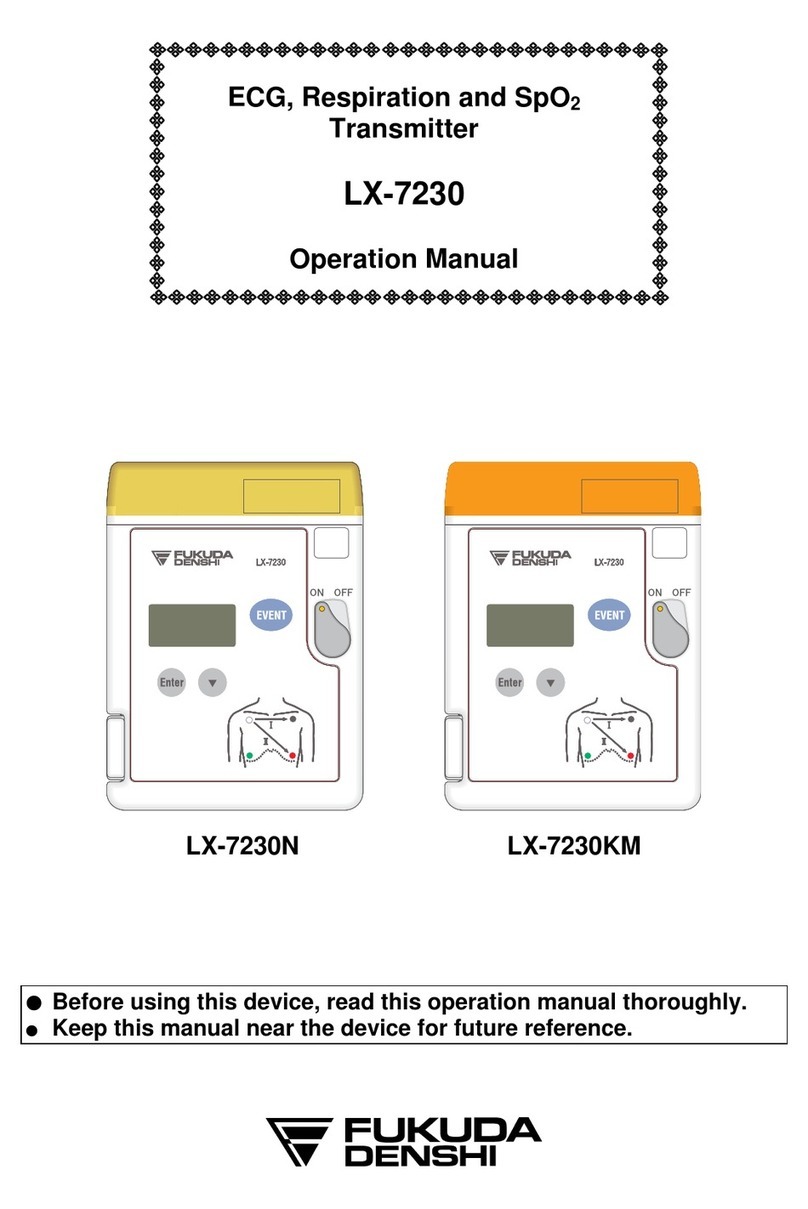
iv
5. After using the equipment, verify the following items.
Make sure to turn off the power of the equipment.
When unplugging the cables, do not apply excessive force on the
cable and pull from its connector.
Clean the accessories and cables, and keep them together in one
place.
Keep the equipment clean to ensure proper operation for the next
use.
Make sure to remove the batteries if the equipment is not used for a
long time. The leakage from the batteries may damage the
equipment or an explosion from the batteries may occur.
6. If the equipment is damaged and in need of repair, ensure patient
safety by immediately turning the equipment off and remove the
electrodes and/or probe from the patient. User should not
attempt service. Label the unit “OUT OF ORDER” and contact
Fukuda Denshi representative.
7. Do not remodel the equipment.
8. Maintenance Check
Make sure to periodically check the equipment, and accessories.
Before reusing the equipment that has been left unused for a while,
make sure that the equipment works normally and safely.
9. When using electrosurgical knives or defibrillator with this
equipment, take care of the following.
To prevent burn in ury to the patient, verify proper attachment of
patient ground plate, ECG electrode type for the electrosurgical
knives, and the quantity of gel, output energy for the defibrillator.
Also, verify that a proper ground is selected.
Some types of equipment other than the above may cause
accidental hazards to the patient and operator due to the conditions
of the equipment
.
Read the operation manual attached to each
equipment and understand the precautionary instructions prior to
use.
Non-E plosion Proof
DANGE R
Never operate the equipment in the presence of flammable anesthetics,
high concentration of oxygen. It may cause an explosion or fire.
Never operate the equipment inside a hyperbaric chamber. It may
cause an explosion or fire.
Never operate the equipment where flammable gas or fluid such as
anesthetic, oxygen, and hydrogen are used. It may cause an explosion
or fire.