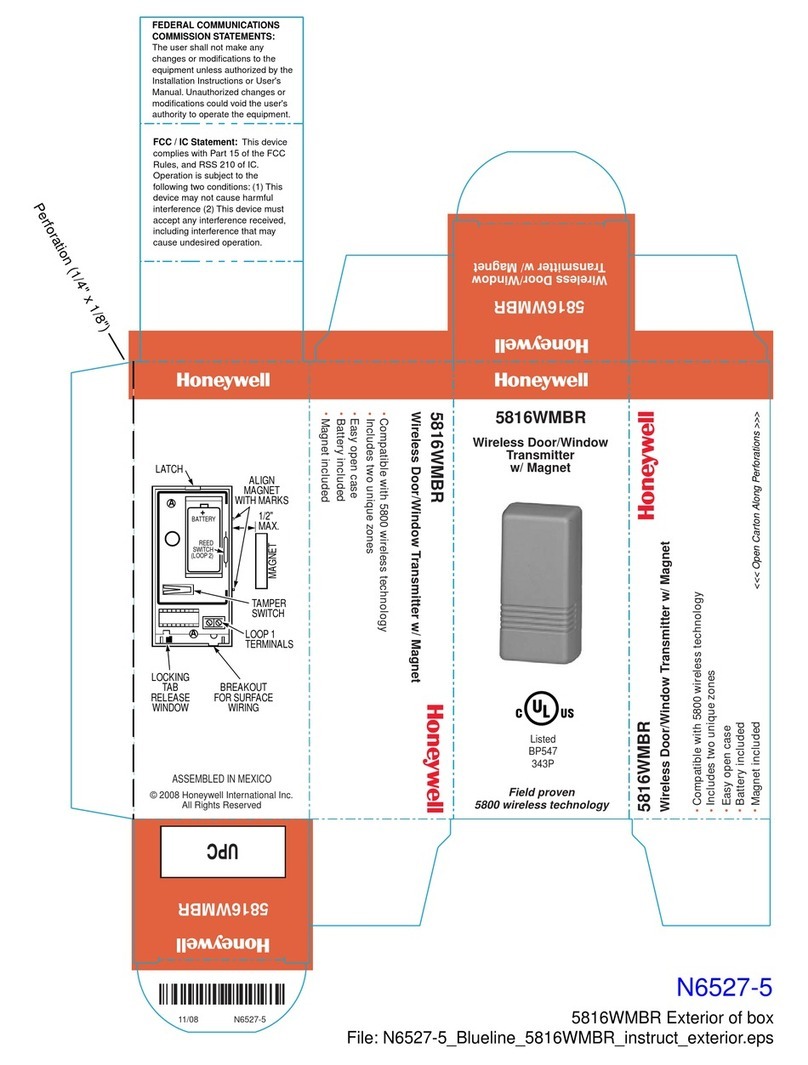
v
Precautions for Safe Operation of Medical Electrical Equipment
Cautions described here are regarding the general instructions for safety use
to the patient and users. As for cautions about the LX-7230N/LX-7230N(G),
please refer to the following pages.
CAUTION
1. Users should have a thorough knowledge of the operation before
using this equipment.
2. Pay attention to the following when installing or storing the
equipment.
Do not install or store in an area where the equipment will be subject to
splashing water.
Do not install or store in an area where the environmental conditions, such
as atmospheric pressure, temperature, humidity, ventilation, sunlight, dust,
sodium, sulfur, will adversely affect the system.
Place the equipment on a stable surface where there is no inclination,
vibration, or shock (including during transportation).
Do not install or store in an area where chemicals are stored or gases are
evolved.
3. Before operating the equipment, verify the following items.
Check the cable connection and polarity to ensure proper operation of the
equipment.
Ensure that all cables are firmly and safely connected. Especially, recheck
the attachment and connection condition of electrode and the probe
(sensor).
Pay special attention when the equipment is used in conjunction with other
equipment as it may cause erroneous judgment and danger.
Check the remaining battery level.
When replacing the battery, make sure that the battery polarity is correct.
Do not charge the battery.
4. During operation of the equipment, verify the following items.
Do not operate the equipment beyond the time period required for
diagnosis and medical care.
Do not pick up and/or swing the equipment pulling/grabbing the probe
(sensor) or cable part. It may damage the equipment and lead to
measurement error.
Always observe the equipment and patient to ensure safe operation of the
equipment.
If any abnormality is found on the equipment or patient, take appropriate
measures such as ceasing operation of the equipment and/or detaching
the probe (sensor) and/or electrode, in the safest way for the patient.
Do not allow the patient to come in contact with other equipments.