7
batteries. Replace with 4 new batteries at the same time. Long-life alkaline batteries are
recommended.
2. To prevent the damage of monitor from leaked battery fluid, please take out of battery if the monitor
unused in a long time (generally more than 3 months). If battery fluid should get in your eyes,
immediately rinse with plenty of clean water. Contact a physician immediately.
3. Dispose of the device, components and optional accessories according to applicable local regulations.
Unlawful disposal may cause environmental pollution.
4.2 System Settings
After you load the battery or connect power for the monitor, long press the SET button for more than 3s,
and then you can start to set.
Setting the User:
Press the MEM button to select User A or User B. When display A (/B) on the screen, press the MEM
button to switch to user B (/A). Press the SET button to confirm.
Setting the Year:
When the year display is flashing, press the MEM button continuously and it will increase continuously 1
by 1 until 2049, and then return the original year , once the year set is OK, press SET button to confirm.
Setting Month/Date:
Initial Month/Date is 1/01, when the Month display is flashing, press the MEM button, the month will
increase by 1, press SET button to confirm, and do in the same way to set the date. Press SET button to
confirm.
Setting Time:
When the hour display is flashing, press the MEM button, the hour will increase by 1, press SET button to
confirm, and do in the same way to set the minute. Press SET button to confirm.
Record Delete:
When you checking the memory data, long press MEM button to delete existing user measurement data.
Note:
If you decide to delete all the records, please keep the records in another way, in case you need it some
days later. Take the battery out won’t lead to a record missing.
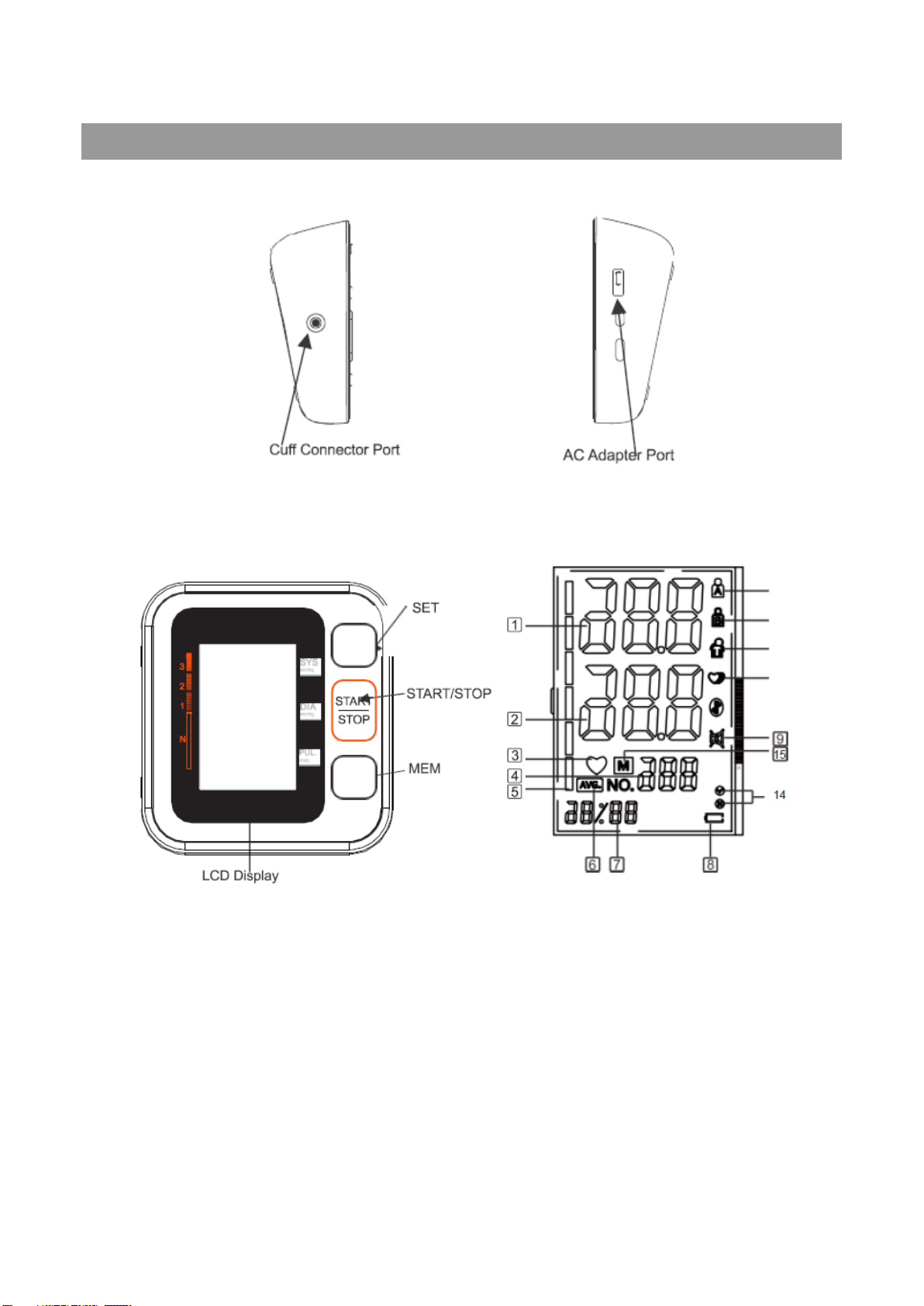
4.3 Cuff tube connection
Insert the cuff tube into the opening on the left side of the monitor indicated by the drawing of a cuff.