6
1.3. Key Features
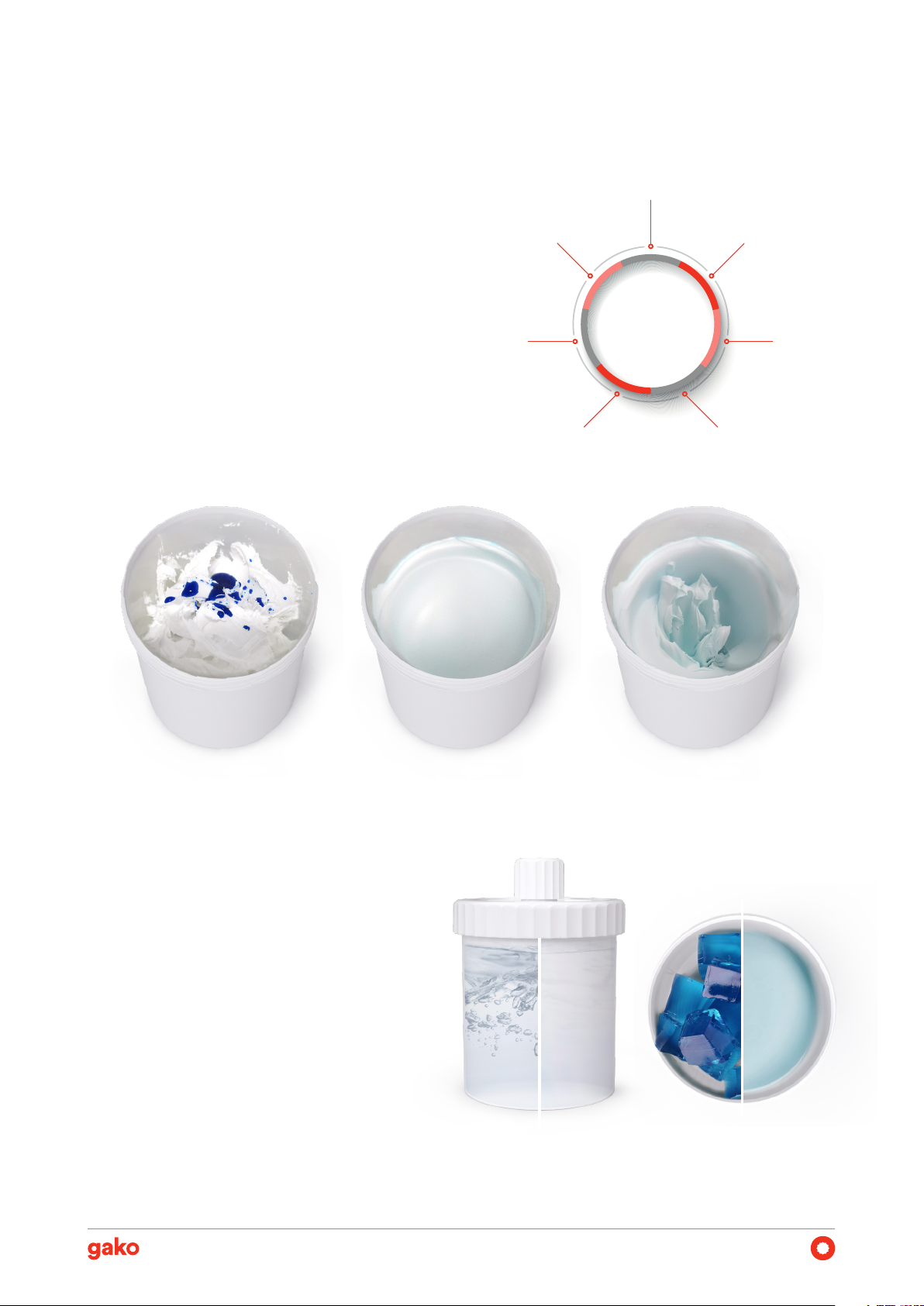
Single-Step Process
The gako PM140 combines mixing, melting, and deaer-
ation processes in a single step. Therefore, it simpliies
the compounding of pharmaceutical preparations and
saves time while ensuring high-quality formulations. As
demonstrated in studies conducted by our scientiic ex-
pert team, the temperature rise is limited to 45°C, mak-
ing it suitable for most heat-sensitive ingredients.
Time-Saving
The high mixing speed of gako PM140 is ixed to 2800
rpm, allowing the preparation of formulations in less than
60 seconds.
Dosage Accuracy
In volume-dependent doses, as in HRT creams, a reliable
mixing process that deaerates the formulation improves
API distribution and ensures dosage accuracy for each
application.
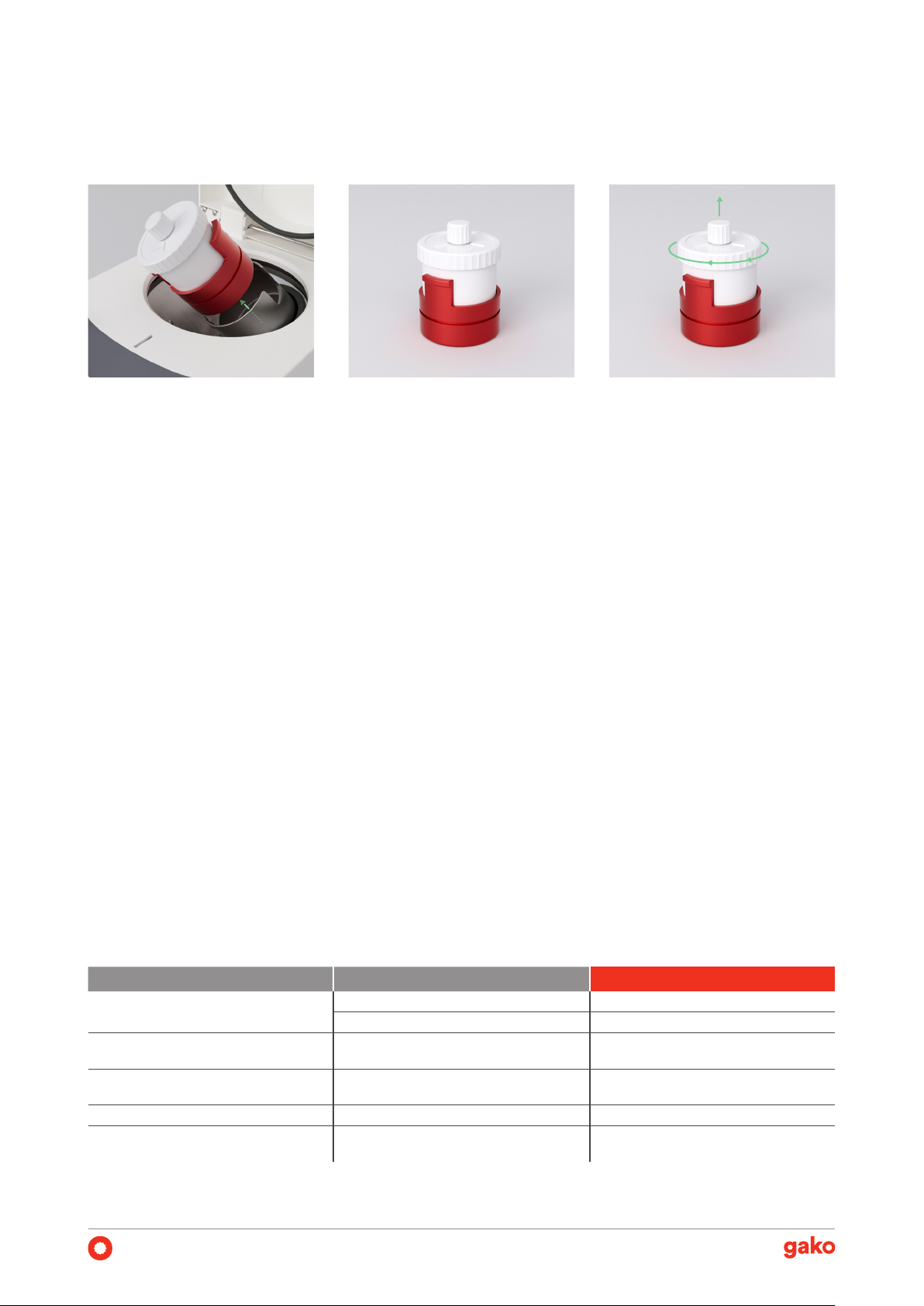
Material-Loss Prevention
In traditional mixing processes, material loss occurs dur-
ing the transfer to the inal package after mixing. Even if
the exact amounts are calculated and weighed, the com-
pounded preparation is never completely transferred
from the mortar into the package, especially when work-
ing with semi-solid dosage forms. To prevent material
loss, the gako PM140 is designed to mix the formulation
in a disposable PM jar, also used as a inal package to be
delivered to the patient.
Conservation of Resources
The gako PM140 contributes to water conservation, as it
does not require subsequent washing and rinsing steps
of spare parts such as blades and mixing rods.
Low-Maintenance
The gako PM140 is distinguished by quality materials
that allow for a low-maintenance and durable service,
improving cost-eiciency. Due to its compact and func-
tional design, the device can be easily integrated into lab
furniture or used on the workbench. It also eliminates
issues when compounding colored ingredients, such as
coloring the blades or wearing them out by changing
color and avoiding cross-contamination in a hormone
preparation through the edges.
Compounding Hazardous Drugs
The term “hazardous drug” (HD) was irst described by
the American Society of Health-System Pharmacists
(ASHP) in 1990 and has also been used by Occupational
Safety and Health Administration (OSHA) for compounds
that display the following characteristics: genotoxicity;
carcinogenicity; teratogenicity or loss of fertility; and
severe toxic manifestations at low doses in experiments
with animals or treated patients. An API is considered
hazardous if it features one or more of these characteris-
tics, and new APIs with structure and toxicity proiles that
mimic those of hazardous APIs are also classiied as HDs.
According to the United States Pharmacopoeia (USP),
pharmacists can be potentially exposed to HDs while
compounding when:
• Weighing or mixing components;
• Crushing or splitting tablets or opening capsules;
• Pouring oral or topical liquids from one container
to another;
• Constituting or reconstituting powdered or
lyophilized HDs;
• Withdrawing or diluting injectable HDs from
parenteral containers;
• Expelling air or HDs from syringes;
• Contacting HD residues present on Personal Protective
Equipment (PPE) or other garments;
• Deactivating, decontaminating, cleaning, and
disinfecting areas contaminated with or suspected
to be contaminated with HDs;
• Maintenance activities for potentially
contaminated equipment and devices.
The gako PM140 decreases the risk of exposure to HDs
as it works in a closed environment provided by the PM
jars. Additionally, the PM jars can be placed in negative
pressure cabinets, which increases safety for the com-
pounding of HDs.
Volume-Adjustable Jar
For the preparation of smaller batches than 100 mL, the
gako PM jar 100mL HV enables the reduction of the jar
volume by pushing the bottom upwards after the op-
eration. This eliminates the requirement of acquiring
multiple jar sizes and additional jar holders/adaptors to
accommodate them with the device. Additionally, it min-
imizes air contact by removing excess space, resulting in
improved quality.