Talk Forward...........................................................................................................................................30
Interlock ..................................................................................................................................................30
Tracking ..................................................................................................................................................30
Status / Audiogram Button......................................................................................................................30
Data Transfer...........................................................................................................................................30
Printing....................................................................................................................................................31
Stimulus Channel 1 .................................................................................................................................34
Stimulus Channel 2 .................................................................................................................................34
Transducer Output Selector.....................................................................................................................35
Routing Output........................................................................................................................................35
Attenuators (HL Controls).......................................................................................................................36
Channel 1 Present Bar / Interrupt............................................................................................................36
Channel 2 Interrupt Button......................................................................................................................36
Frequency Up / Down .............................................................................................................................36
Data Store................................................................................................................................................36
Navigation Controls ................................................................................................................................37
Scorer / Timer..........................................................................................................................................37
Monitoring...............................................................................................................................................37
Test Type Buttons ...................................................................................................................................37
Function Buttons.....................................................................................................................................38
Chapter 5: Test Type Displays....................................................................................................................39
Monitor....................................................................................................................................................39
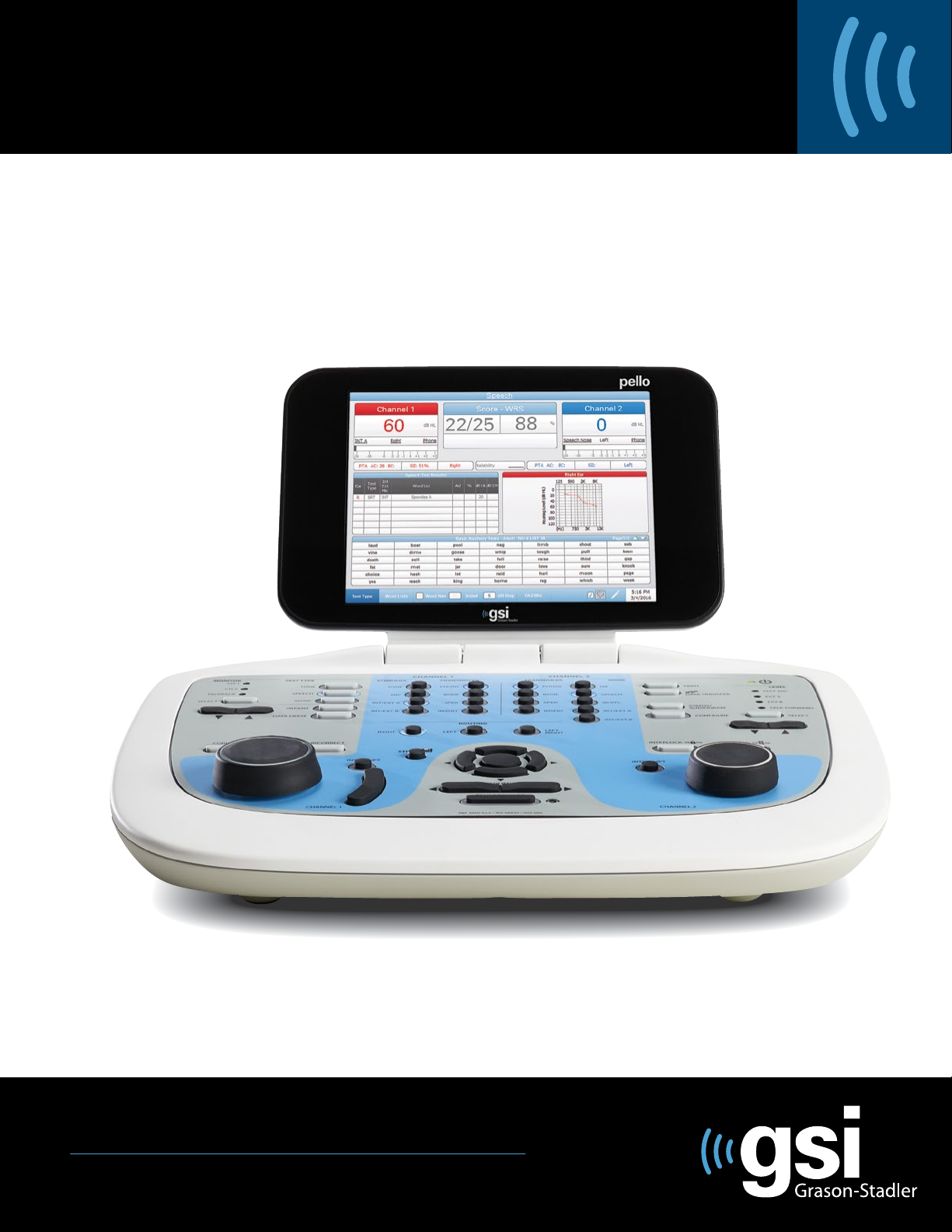
Tone Test Type - Audiogram..................................................................................................................41
Tone Test Type - Status...........................................................................................................................45
Speech Test Type - Status.......................................................................................................................46
Speech Test Type - Audiogram...............................................................................................................51
More Test Type.......................................................................................................................................52
Chapter 6: Operation...................................................................................................................................53
Preliminary Checks .................................................................................................................................53
Typical Evaluations.................................................................................................................................54
Test Type Buttons ...................................................................................................................................54
Routine Test Procedures..........................................................................................................................56
Threshold Determination (Pure Tone): Modified Hughson-Westlake....................................................56
Spondaic Speech Testing, Speech Reception Threshold (SRT)..............................................................57
Word Recognition (PB Words)...............................................................................................................57
Stenger test..............................................................................................................................................58