80834 REV ED 3
CyberWand Dual Ultrasonic Lithotripsy System • Instructions for Use
EN
Return/Repair/Warranty
Return/Repair
All returns must have prior authorization. To comply with OSHA Bloodborne Pathogen Standard 29
CFR 1910.1030 regulations and U.S. Postal and Transportation law, all used medical devices returned
for repair or replacement must be properly cleaned and decontaminated with a chemical germicide that
has been cleared for use as a “Hospital Disinfectant.” To ensure that the product has been properly
decontaminated, a signed Decontamination Certificate should be enclosed in the package.
All medical devices returned to Gyrus ACMI for any reason must be shipped in accordance with Gyrus
ACMI’s return procedures (available upon request) and all applicable regulations. To obtain a return
authorization number (RMA), return address, and instructions, please call Gyrus ACMI Customer Service
at 1-888-524-7266 or 1-763-416-3000.
Please provide the product part number and lot/serial number. Product should be returned in its original
packaging (when possible) and marked with the RMA number on the exterior of the package.
Limited Express Warranty
SHOULD THE PRODUCT BECOME INOPERABLE DURING NORMAL AND PROPER USE IN ACCORDANCE
WITH APPLICABLE INSTRUCTIONS AND WITHIN THE TIME FRAME SPECIFIED BELOW FROM THE DATE
OF SHIPMENT, GYRUS ACMI WILL REPAIR OR REPLACE THE PRODUCT, AT ITS SOLE OPTION, AT NO
CHARGE. GYRUS ACMI MAKES NO OTHER WARRANTIES WITH RESPECT TO THE PRODUCT AND
EXPRESSLY DISCLAIMS ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, AS TO MERCHANTABILITY,
FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER MATTER. IN NO EVENT SHALL GYRUS ACMI BE
LIABLE FOR ANY CONSEQUENTIAL DAMAGES. IN NO EVENT SHALL GYRUS ACMI BE LIABLE FOR ANY
BREACH OF WARRANTY IN ANY AMOUNT EXCEEDING THE PURCHASE PRICE OF THE PRODUCT.
This warranty applies only to the original purchaser and will be voided if the product(s) are serviced or
repaired by anyone other than Gyrus ACMI or an organization duly authorized by Gyrus ACMI for such
purpose.
Ultrasonic Lithotripters ..................................................................1 year
Accessories ............................................................................... 90 days
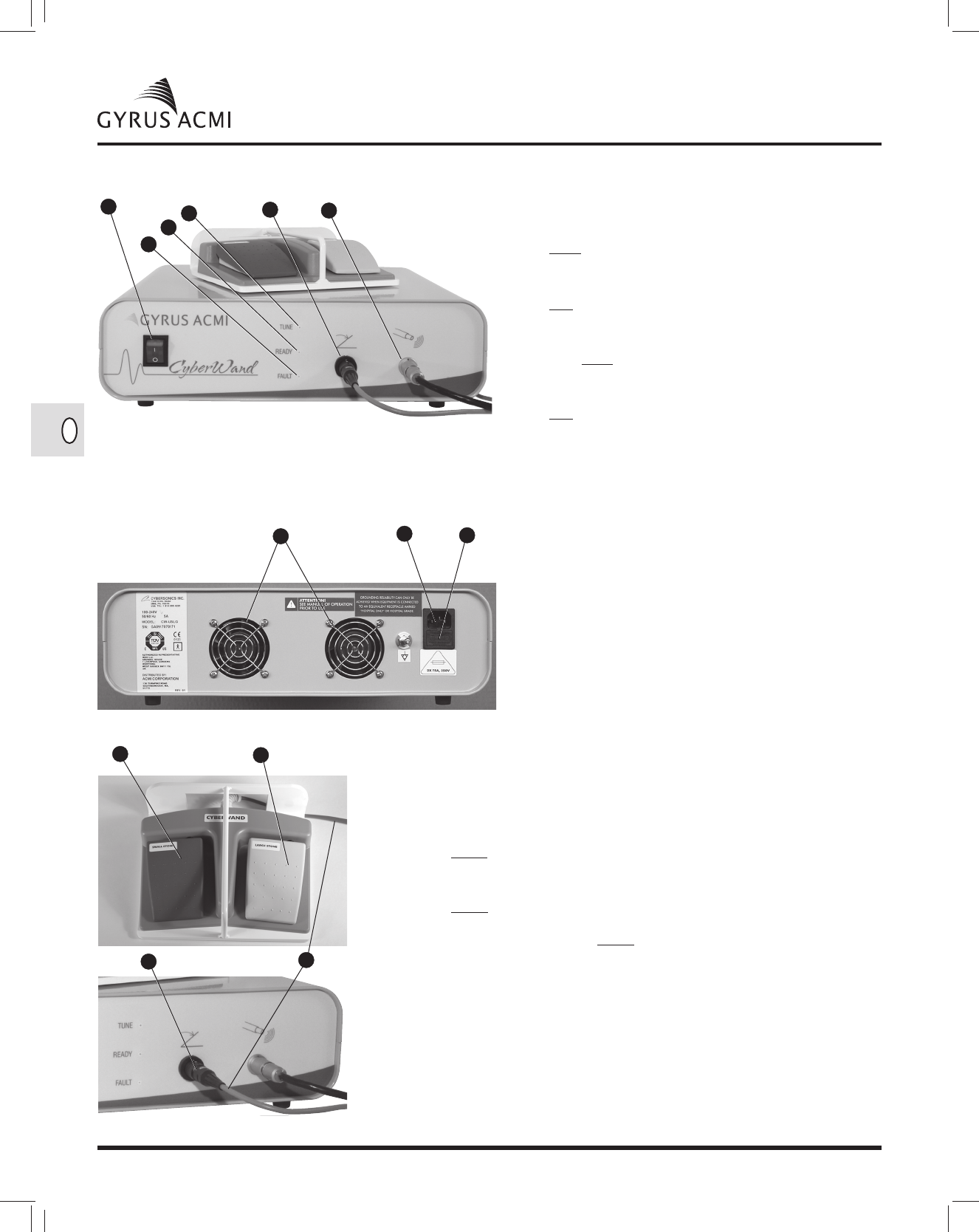
WARNINGS AND CAUTIONS
The following warnings and cautions apply to the use, care, and/or maintenance of the Gyrus ACMI®
CyberWand Ultrasonic Lithotripsy System.
Warnings
(Indicate that danger to life or health can result from misusing the equipment)
1. This device should be operated only by or under the direct supervision of a physician
experienced in ultrasonic lithotripter procedures. The user should be thoroughly familiar with the
operation of this device prior to use.
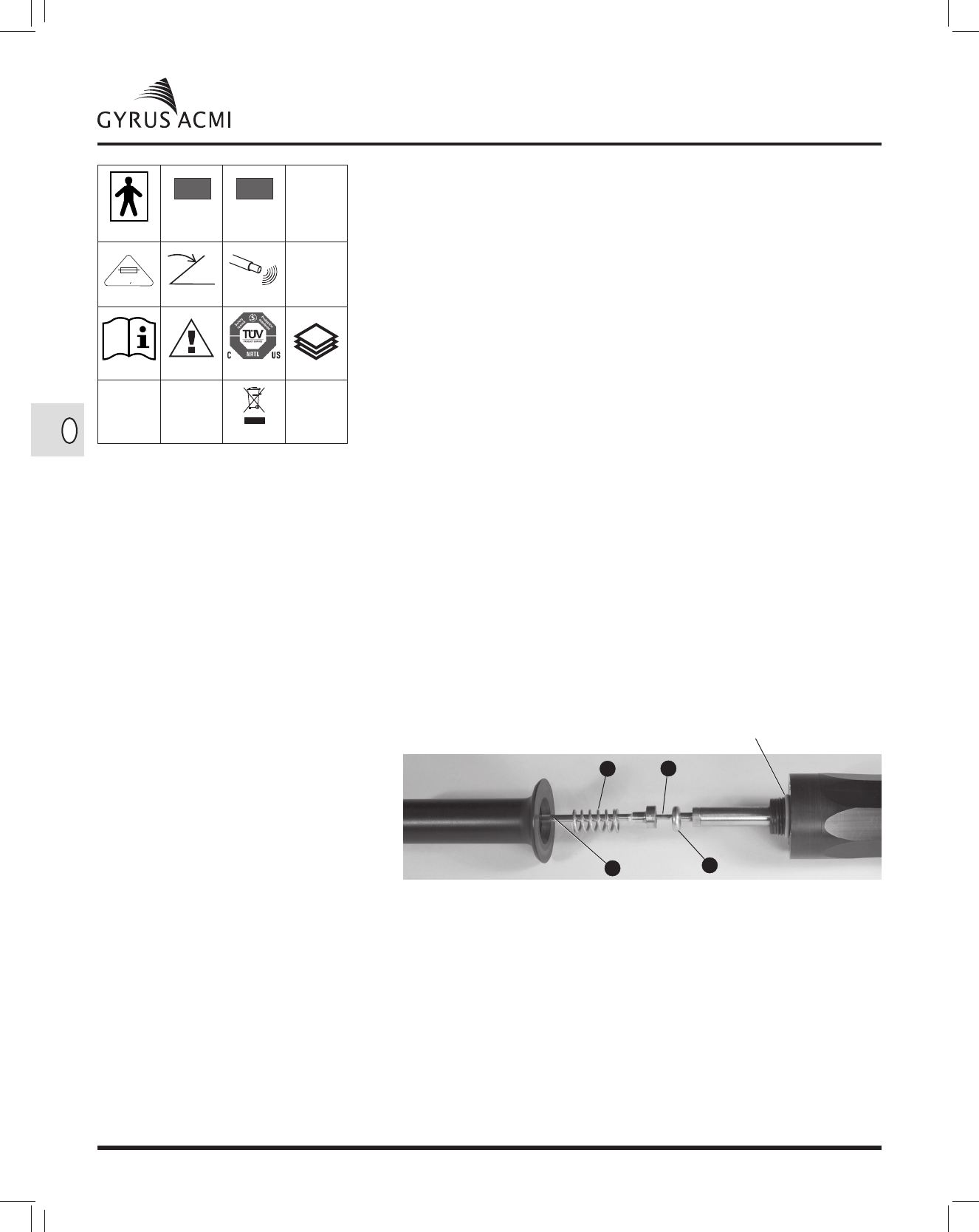
2. This device is designed to function with single-use ultrasonic lithotripsy probes designed
specifically for the CW-USLS generator. Probes are single-use should not be reprocessed for
reuse.
3. Perform the prescribed inspections and operational tests prior to first use and regularly thereafter
to ensure continued satisfactory performance. Do not use a CyberWand Ultrasonic Lithotripter
that fails to meet the criteria stated in the labeling or that has been damaged. Otherwise, injury to
patient, personnel and/or an adverse effect on the procedure could result.
4. When used in an environment containing flammable gasses and high oxygen concentrations, be
aware of the potential for a flammable event. Take routine cautions to prevent this kind of event.
The product is not explosion proof.
5. A potential shock hazard exists when the instrument cabinet is opened. Refer all repairs to
authorized service personnel.
6. Do not adjust electronic circuitry. Contact your local Gyrus ACMI representative or call Gyrus
ACMI customer service at 1-888-524-7266 or 1-763-416-3000.