User Guide HR2-320 (pg 3)
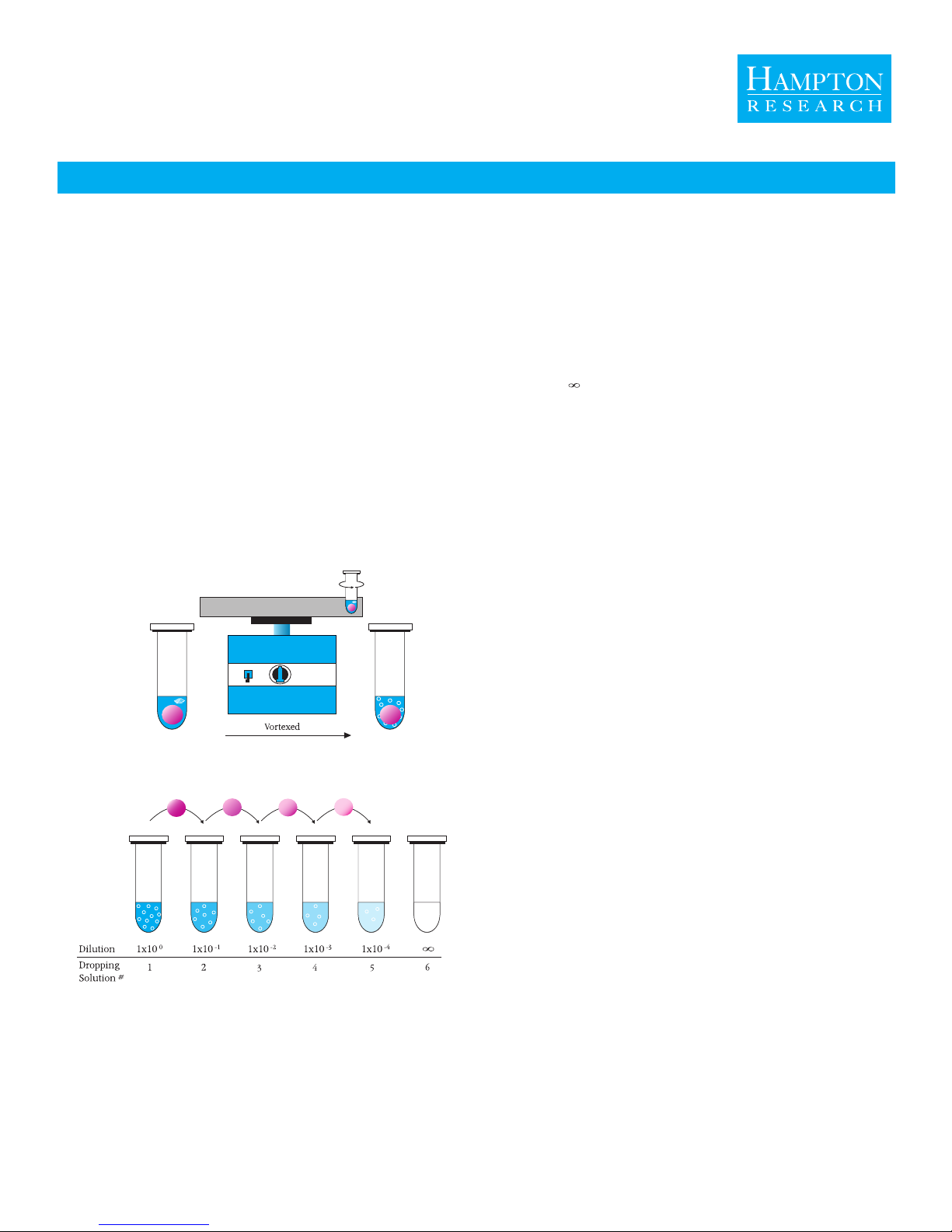
Seed BeadTM
Example 3. The results of Example 2 still produced too many, small crystals
after 24 hours. In an effort to reduce the number of crystals and increase
crystal size, one can use a different serial dilution seed stock. From Preparing
Serial Dilutions for Seeding, use Dropping Solution 2 for the seed stock.
For the crystallization experiment, pipet 1.4 M Ammonium sulfate, 0.07 M
HEPES pH 6.8 into the reagent well (reservoir). For the drop, pipet 1 part of
protein plus 1 part of Dropping Solution 2. The drop will now equilibrate
from 1.0 M to 1.4 M Ammonium sulfate, but with fewer seeds.
Microseed Matrix Seeding (MMS)
Microseed Matrix Seeding is the method where seed crystals are systematically
added to a crystallization screen.5-8 By adding seeds instead of protein:
1. It is likely to greatly increase the number of crystallization hits that are
observed;
2. It is likely that good quality crystals will grow, because the crystals often
grow at lower levels of saturation;
3. Reagents can be used where no spontaneous nucleation takes place, so
that the number of crystals can be controlled by determining the num-
ber of nuclei that are added to the well (by diluting the seed stock).
Setting the Drops for Microseed Matrix Seeding (MMS)
Pipet the crystallization screen reagent into the reagent well (reservoir). To
create the drop, pipet 0.2 ml of crystallization screen reagent (reservoir),
0.1 ml of seed stock from step 5 and 0.3 ml of protein solution. As a starting
point, use the same protein concentration here as used to produce the seeds
in a previous screen. Repeat for the remaining reagents.
Observations, Notes, and Suggestions
1. The 3.0 mm PTFE Seed Bead has a density of 2.2 g/cc.
2. When seeding, one would prefer to have an initial sample / reagent
composition in the drop that will not produce crystals without the addi-
tion of a seed. This will prevent nucleation secondary to the introduced
seeds as well as excessive nucleation.
3. If, after performing the seeding experiment with a particular set of dilu-
tions, one still observes excessive nucleation and small crystals, repeat
the seeding experiment with further dilution of the Seed Stock/Dropping
Solutions.
4. Use a new, clean Seed Bead and microcentrifuge tube each time one
is generating a new seed stock. This will prevent contamination and
carryover.
5. Vortexing the Seed Bead in the presence of detergents and/or other
chemical additives which can foam is not recommended. In the pres-
ence of detergents or other chemical additives which can foam, it is rec-
ommended one use sonication to disrupt the seed using the Seed Bead.
6. Sonication using the Seed Bead allows one to use smaller volumes than
the vortex method.
7. If using sonication do not leave the sample in the ultrasonic bath too
long since this can heat the sample.
8. To prevent splashing when vortexing, grasp the tube in the middle while
vortexing. Should drops of the sample appear on the upper sides of the
tube or in the lid, place the tube in a centrifuge for 3 to 5 seconds to sedi-
ment the liquid. Do not centrifuge for any longer since this will “pellet”
the seeds.
9. Any crystalline protein material can be used for microseeding, including
fine needles, spherulites, microcrystals, irregular poorly-formed crystals
and even granular looking precipitate. Seed anything that might be
crystalline. Skins do not seem to work for seeding.
10. When performing iterative seeding during optimization, be more se-
lective about which seeds to include in the seed stock, identifying and
choosing the best crystals; do not mix the good, bad and the ugly, leave
that to Sergio Leone.
11. Microcrystals in the seed stock are not stable because of the lower protein
concentration in solution. The seed stock should be kept on ice and
frozen as soon as possible, preferably at -80ºC when not being used.
12. It has been observed that, for some proteins, only fresh crystals work for
seeding. Crystals that have been in the lab for a few weeks may not work
for seeding, even though the crystals still diffract. Make a seed stock as
soon as possible after the crystals stop growing.
13. One may consider combining as many hits as possible to make the seed
stock. But be careful to avoid creating solutions that could result in
salt crystals or phase separation. One could try to combine the drops
from all the hits in PEG based conditions to make one seed-stock, and
the drops from all the hits in salt based reagent to make another seed
stock. Avoid mixing metals and salts such as calcium and phosphate
as this can produce salt crystals. Avoid mixing high salt and high PEG
concentration as this can produce phase separation.
14. MMS experiments can be dispensed by manually. The volumes dis-
pensed will be increased slightly to approximately and in the following
order, 1.5 µl of protein, 1 µl of reservoir solution, and 0.5 µl of seed stock.
15. When using automation for MMS, contact dispensing appears to be fa-
vored as non-contact dispensing can be prone to clogging.
16. Be careful not to optimize salt crystals. Salt crystals are a side effect of
MMS due to the random mixing of reagents.
17. The potential of seeding and MMS. Increase hit rate in crystallization
screens. More reagents to choose from for ligand soaking and heavy
atom derivatives. New space groups. Use apo form to seed for ligands
and inhibitors. Avoid twinning. Larger, more ordered, better diffracting
crystals. Cross seeding between complexes.
Solutions for Crystal Growth