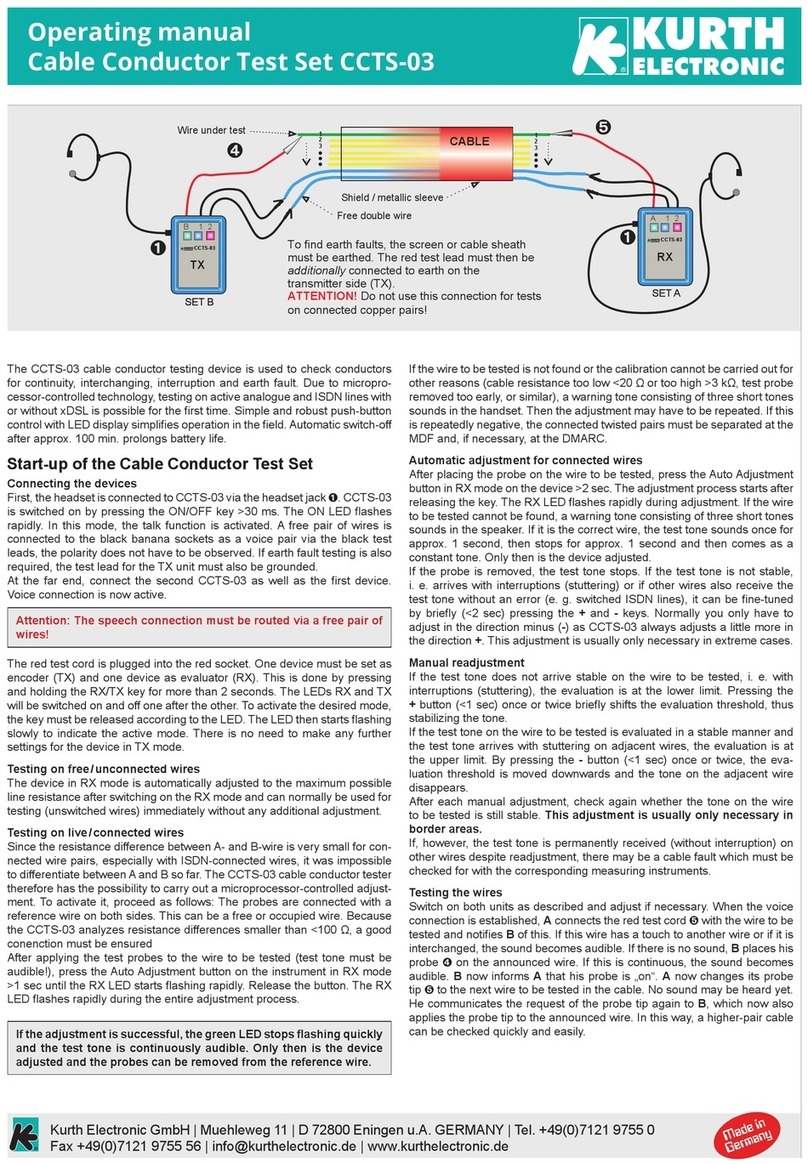
9 | P a g e
IFU-0001 Rev B 01/17
W RR NTY REGISTR TION
To ensure your warranty is in force, lease com lete and mail,
or fax your warranty card to Hoggan Scientific LLC at 800-915-
3439. Or visit www.hogganhealth.net to register your warranty
information online. Please save roof of your original urchase
date, such as your sales sli , invoice, credit card voucher, or
cancelled check to establish the warranty eriod.
W RR NTY REP IRS
Before deciding that your microFET2™ is ino erable or
defective, lease review and follow the information in this
instruction booklet.
In the unlikely event your microFET2™ becomes ino erable,
lease contact Hoggan Scientific, LLC to arrange to have the
equi ment re aired. Hoggan reserves the right to re air or
re lace the unit with new or refurbished arts or equi ment.
Hoggan's Customer Service De artment can be contacted at
800-678-7888, or by email at contact@hogganhealth.net. When
Hoggan Customer Service Re resentative authorizes return of
the roduct, you will be given Return Merchandise
Authorization (RMA) number. Please include the RMA number
with your unit. For confirmed warranty re airs, the customer is
res onsible for the a licable shi ing costs and shi ing to
Hoggan Scientific.
W RR NTY EXCLUSIONS ND LIMIT TIONS
The microFET2™ warranty does not cover damage by
negligence, misuse, or accident. Damage or unit failure caused
by modifications or re airs other than those a roved by
Hoggan or its authorized re air agent, or damage to equi ment
resulting from im ro er installation or o eration is not covered.
Any warning or instructional labels or decals must remain on
the unit for the warranty to be valid.
This warranty a lies to the original urchaser. Some states do
not allow the exclusion or limitation of incidental or
consequential damages, in which case the exclusions and
limitations may not a ly. This warranty gives s ecific legal
rights, and may also have other rights, which vary from state to
state. To determine the legal rights in your state, consult your
local or state consumer affairs office or State Attorney General.
CUSTOMER SERVICE REP IRS
Customer satisfaction is im ortant to Hoggan. We are ha y to
assist with questions, roblems or service issues on any Hoggan
roducts you may own. Our business has grown on the basis of
excellent roduct quality and customer satisfaction. Our
fulltime customer service re resentatives are available from
7:00 am to 4:30 m MST at 800-678-7888 to meet your needs.
You can also contact Hoggan Scientific online regarding your
customer service issue or calibration needs by e-mailing us at
contact@hogganhealth.net.
ORDERING REPL CEMENT P RTS
Hoggan Products are manufactured to exacting s ecifications.
When re lacing worn or damaged arts, use only original arts
su lied by Hoggan Scientific. The use of substitute or
unauthorized arts will void your warranty and may increase
the ossibility of injury to the user, or cause additional damage
to the unit.
When ordering Re lacement Parts, lease take the unit out of
service, and com lete the following:
• Identify the brand, model, and serial number, and note
the unit's function.
• Identify and document the roblem and the worn or
missing arts.
• Contact Hoggan Scientific LLC. Re lacement arts
(attachments) will be shi ed directly from Hoggan.
All re air services will be erformed at Hoggan Scientific LLC
Manufacturing lant.
Exce t for re lacing batteries, do not attem t to re air the unit
on your own. This will void all warranties.
microFET2™ batteries, re lacement arts and Preferred Service
Contracts can be ordered either by calling Hoggan Scientific LLC
or order online at www.hogganhealth.net.
microFET2™ SPECIFIC TIONS
• Weight: 1 lb.
• O eration Use Time:
o Non-wireless mode - 90 hours continuous
o Wireless mode - 6 hours continuous
• Trans ortation, Storage, and O erating Conditions:
o Tem erature: 11 – 33 degrees Celsius (52 – 92
degrees Fahrenheit)
o Humidity: 30 - 80% humidity non-condensing
o Atmos heric Pressure: 800 hPA - 1060 hPA.
(11.60 si – 15.37 si)
• Maximum Force Ca acity: 300 lbs. (136 kgf / 1320
Newtons)
• Internal Power Source - Battery: Model ICR14250 user
serviceable, 3.7 volt 1/2 AA lithium ion rechargeable
battery 280 mAH.
• In ut Power: 5V 1.0A
• Recharge Time: Three (3) continuous hours of
charging
• Power Su ly: Phihong Model PSAC05R-050-L6. In ut
- 100-240V. Out ut – 1A. 5 volt DC regulated
• No Protection Against Harmful Ingress of Water: IPX0
– ordinary equi ment
• Test Range:
o Low Threshold 0.8 lbs to 300 lbs in 0.1 lb
increments Metric Newtons: 3.6N 1320N in
0.4N increments KGF (kilograms force):
o 0.4kgf to 135kgf in .1kgf increments